Fig. 2 |. Structural insights into cry2 inactivation.
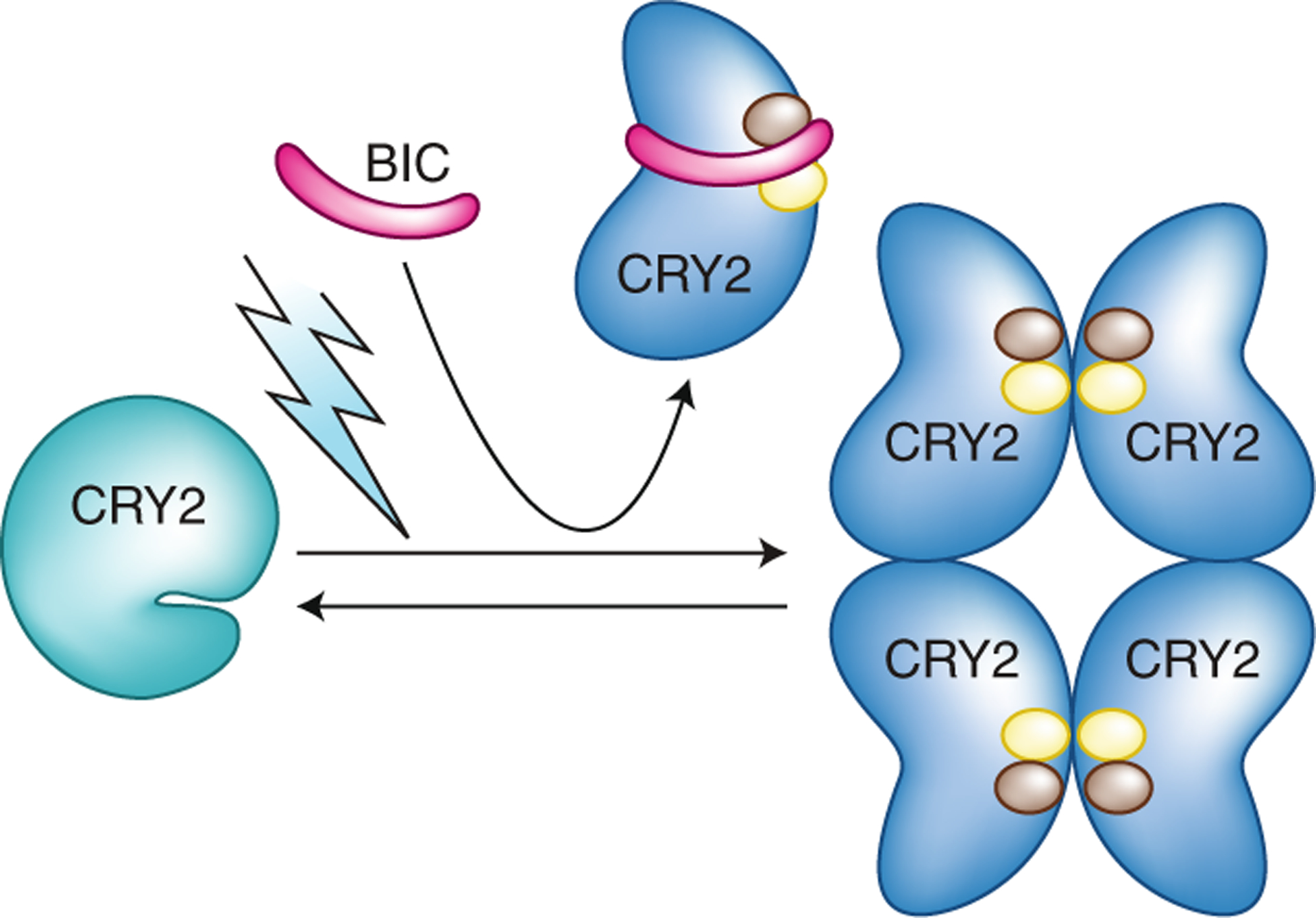
BIC displays a ‘waist belt’ structure, which interacts with photoexcited CRY2 and wraps around the PHR domain to prevent CRY2 dimerization. The present structural analyses11,12 reveal that at least two residues of CRY2, R208 and W349, represented by brown and yellow circles, are located at the interfaces of both the CRY–CRY complex and the CRY–BIC complex. This finding suggests that BIC acts as a competitive inhibitor, binding CRY2 to block CRY photo-oligomerization and photoactivation.
