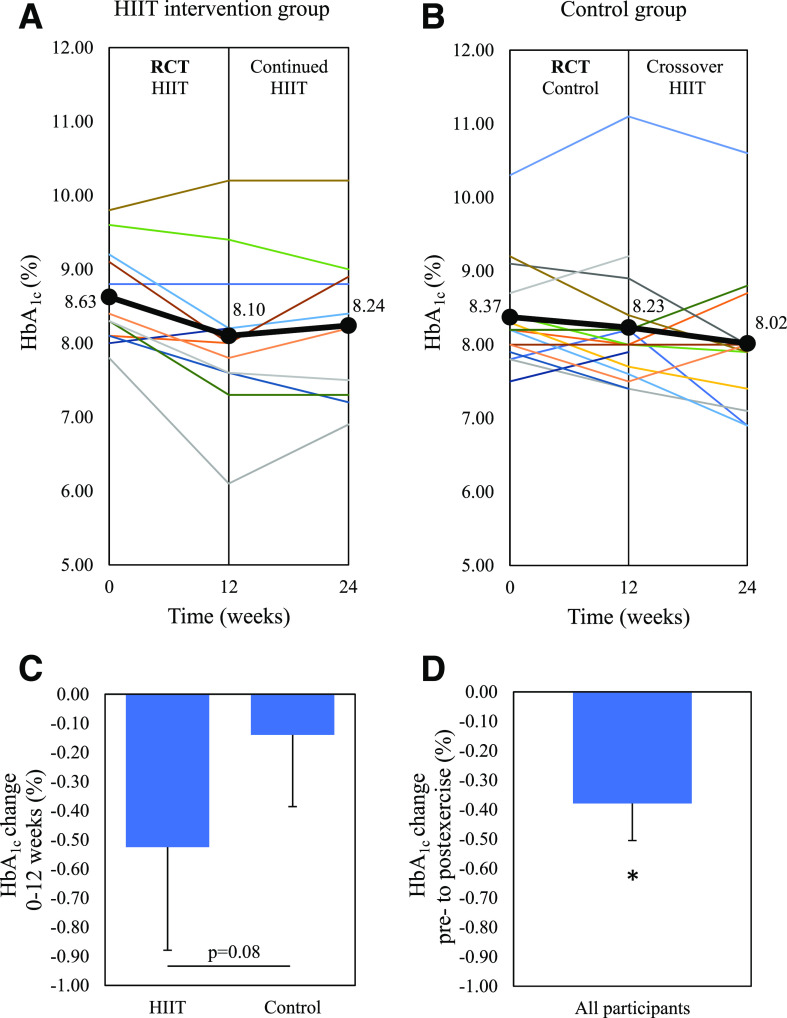
Figure 2.
The change in HbA1c for individual participants. A: HIIT group where 0–12 weeks involved the HIIT intervention, and 12–24 weeks involved continued, unsupervised HIIT. B: Control group where 0–12 weeks involved inactive control, and 12–24 weeks involved the HIIT intervention as the crossover design. C: Change in HbA1c from 0 to 12 weeks between the HIIT and control groups as the primary study end point. D: Predefined secondary end point for change in HbA1c from preexercise to postexercise for all 24 participants who undertook 12 weeks of HIIT. Bold line indicates the mean HbA1c. Data are mean ± SEM. *P < 0.05 change from preexercise. RCT, randomized controlled trial.