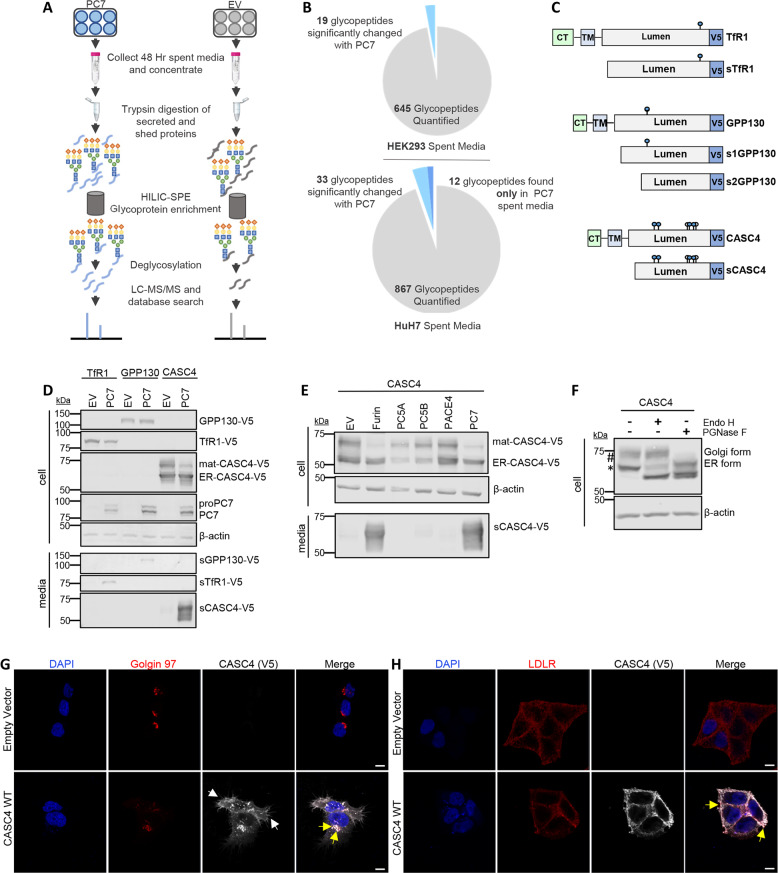
Fig. 1. Mass spectrometry identifies two type-II transmembrane proteins shed by PC7 and Furin.
a Schematic representation of the mass spectrometry strategy. The media from HEK293 or HuH7 cells overexpressing PC7 or empty vector were analyzed by LC-ESI-MS/MS and screened for quantitative changes in N-glycosylated soluble proteins. b Quantitative changes of 645 and 867 enriched glycosylated tryptic peptides from the spent media of HEK293 and HuH7 cells. c Schematic representation of human transferrin receptor 1 (TfR1), Golgi Phosphoprotein of 130 kDa (GPP130), and Cancer Susceptibility Candidate 4 (CASC4) identified in the analysis. Depicted are the cytosolic tail (CT), the transmembrane domain (TM), the luminal domain, and the C-terminal V5-tag. The blue circles are depicting potential N-glycosylation sites and the white circles are depicting potential O-glycosylation sites. d Western blot analysis of cell lysates and media from HEK293 cells expressing TfR1-V5, GPP130-V5, or CASC4-V5, with either empty pIRES-empty vector or hPC7. e Western blot analysis of cell lysates and media from HEK293 cells expressing CASC4-V5 with all the basic aa PCs. f Western blot analysis of cell lysates from HEK293 expressing CASC4-V5 treated with endo H or PGNase F. g Immunofluorescence analysis of permeabilized HeLa cells overexpressing CASC4-V5 colocalizing (yellow arrows) with Golgin-97, or in non-permeabilized cells with LDLR (h). These results are representative of three independent experiments. Scale: 10 µm.