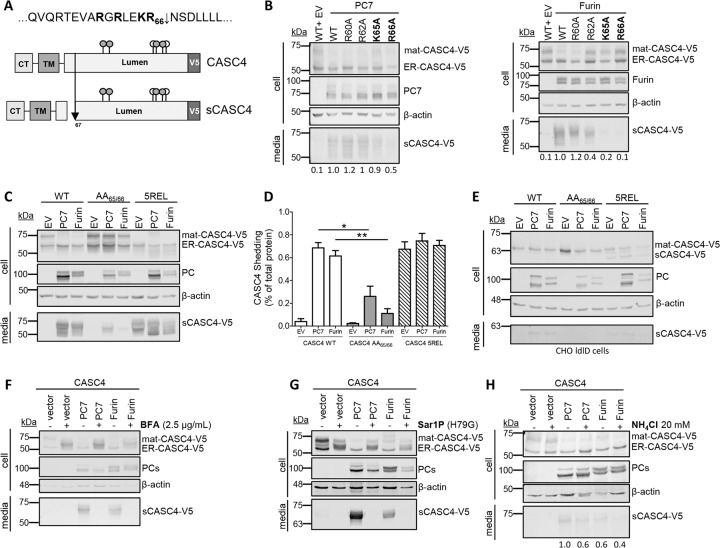
Fig. 2. CASC4 cleavage by PC7 and Furin occurs after Arg66↓ in acidic compartment.
a Schematic representation of the predicted cleavage site KR66↓ generating a N-terminal fragment and a luminal domain. The gray circles are depicting potential N-glycosylation sites and the white circles are depicting O-glycosylation sites. b Western blot analysis of cell lysates and media from HEK293 cells overexpressing CASC4-V5 WT and different point mutation (R60A, R62A, K65A, or R66A) co-expressed with either pIRES-empty vector or with hPC7 or hFurin. c, d Western blot analysis and quantifications of cell lysates and media from HEK293 cells overexpressing CASC4-WT-V5, CASC4-5REL-V5 optimally cleaved mutant, or a PC-non-cleavable AA65/66 site, co-expressed with either pIRES-empty vector or with hPC7 or hFurin. e Western blot analysis of cell lysates and media from CHO-ldlD cells overexpressing CASC4-V5 WT, CASC4-5REL optimally cleaved mutant or a PC-non-cleavable AA65/66 site, co-expressed with either pIRES-empty vector or with hPC7 or hFurin. f Western blot analysis of cells lysates and media from HEK293 cells overexpressing CASC4-V5 WT, pIRES-empty vector, and hPC7 or hFurin treated with Brefeldin A (2.5 µg/ml) or NH4Cl (20 mM) (g). h Western blot analysis of cell lysates and media from HEK293 cells overexpressing CASC4-V5 WT, pIRES-empty vector, dominant-negative Sar1P-(H79G), and/or with hPC7 or hFurin. These results are representative of three independent experiments. Error bars indicate averaged values ± standard error from the mean (SEM). P-values: *P ≤ 0.05, **P < 0.01, (two-sided Student’s t-test).