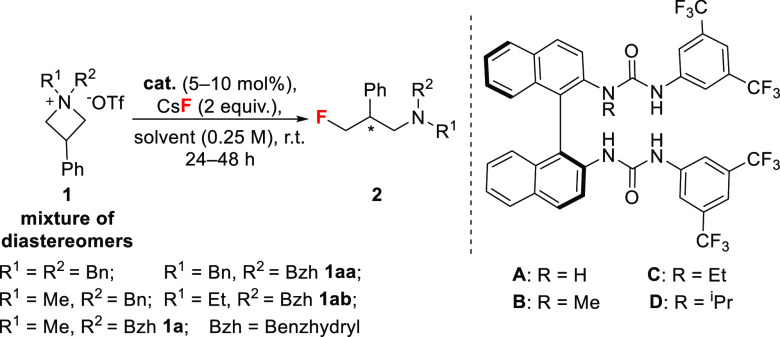
Table 1. Optimization of the Reaction Conditionsa.
| entry | R1 | R2 | cat. | solvent | yieldb | e.r.c |
|---|---|---|---|---|---|---|
| 1 | Bn | Bn | A | CH2Cl2 | traces | – |
| 2 | Me | Bn | A | CH2Cl2 | traces | – |
| 3 | Me | Bzh | A | CH2Cl2 | 14% | 55:45 |
| 4 | Me | Bzh | B | CH2Cl2 | 20% | 55:45 |
| 5 | Me | Bzh | C | CH2Cl2 | 20% | 75:25 |
| 6 | Me | Bzh | D | CH2Cl2 | 45% | 74:26 |
| 7 | Me | Bzh | D | CHCl3 | 56% | 67:33 |
| 8 | Me | Bzh | D | 1,2-DFB | 47% | 79:21 |
| 9 | Me | Bzh | D | 1,2-DCE | 51% | 81:19 |
| 10 | Et | Bzh | D | 1,2-DCE | 40% | 96:4 |
| 11d,e | Et | Bzh | D | 1,2-DCE | 93% | 96:4 |
| 12 | Bn | Bzh | D | 1,2-DCE | >95% | 96:4 |
| 13d,f | Bn | Bzh | D | 1,2-DCE | 98% | 96:4 |
Reaction conditions: 0.05 mmol of 1, 0.25 M, 10 mol% cat., stirring at 900 rpm, 24 h.
Determined by 19F NMR spectroscopy with 4-fluoroanisole as an internal standard.
Enantiomeric ratios were determined by HPLC using a chiral stationary phase.
Yield of isolated product.
72 h, 10 mol% cat.
48 h, 5 mol% cat.