Scheme 2. Synthesis of 13a1–6, 13b1–6, 13c1–6, and 13d1–6a.
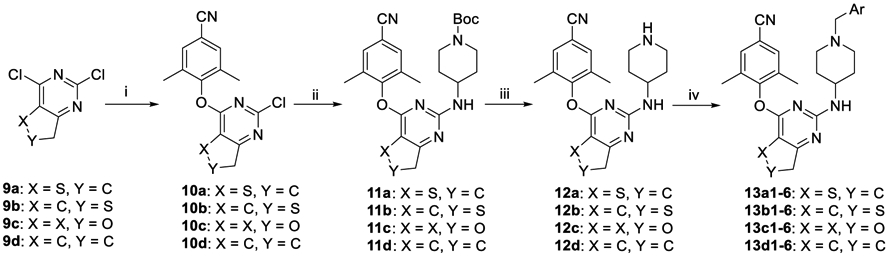
aReagents and conditions: (i) 3,5-dimethyl-4-hydroxybenzonitrile, DMF, K2CO3, rt; (ii) N-(tert-butoxycarbonyl)-4-aminopiperidine, DMF, K2CO3, 120 °C; (iii) TFA, DCM, rt; (iv) substituted benzyl chloride (or bromide) or 4-picolyl chloride hydrochloride, DMF, K2CO3, rt.
