Abstract
Background:
Trauma causes tissue injury that results in the release of damage associated molecular patterns (DAMPs) and other mediators at the site of injury and systemically. Such mediators disrupt immune system homeostasis and may activate multicellular immune responses with downstream complications such as the development of infections and sepsis. To characterize these alterations, we used time-of-flight mass cytometry to determine how trauma plasma affects normal peripheral blood mononuclear cell (PBMC) activation to gain insights into the kinetics and nature of trauma-induced circulating factors on human immune cell populations. A better understanding of the components that activate cells in trauma may aid in the discovery of therapeutic targets.
Methods:
PBMCs from healthy volunteers were cultured with 5% plasma (healthy, trauma-1day, or trauma-3day) or known DAMPs for 24 hours. Samples were stained with a broad immunophenotyping CyTOF antibody panel. Multiplex (Luminex) cytokine assays were used to measure differences in multiple cytokine levels in healthy and trauma plasma samples.
Results:
Plasma from day 1, but not day 3 trauma patients induced the acute expansion of CD11c+ NK cells and CD73+/CCR7+ CD8 T cell subpopulations. Additionally, trauma plasma did not induce CD4+ T cell expansion but did cause a phenotypic shift towards CD38+/CCR7+ expressing CD4+ T cells. Multiplex analysis of cytokines by Luminex showed increased levels of IL-1RA, IL-6 and IL-15 in trauma-1day plasma. Similar to trauma day 1 plasma, PBMC stimulation with known DAMPs showed activation and expansion of CD11c+ NK cells.
Conclusions:
We hypothesized that circulating factors in trauma plasma would induce phenotypic activation of normal human immune cell subsets. Using an unbiased approach, we identified specific changes in immune cell subsets that respond to trauma plasma. Additionally, CD11c+ NK cells expanded in response to DAMPs and LPS, suggesting they may also be responding to similar components in trauma plasma. Collectively, our data demonstrate that the normal PBMC response to trauma plasma involves marked changes in specific subsets of NK and CD8+ T cell populations. Future studies will target the function of these trauma plasma reactive immune cell subsets. These findings have important implications for the field of acute traumatic injuries.
Keywords: Trauma plasma, mass cytometry, CyTOF, Immunology, natural killer cells, injury
Background
Despite substantial improvements in the in-hospital care of injured patients, trauma remains one of the leading causes of death worldwide, and this statistic does not include mortalities caused by complications of trauma that may occur after a person survives the initial injury [1–3]. It is well-known that trauma alters the immune system and it is becoming increasingly clear that this process is complex and dynamic, with pro-inflammatory responses developing concomitant with compensatory anti-inflammatory responses. Acting together, these events lead to dysregulated states referred to clinically as the systemic inflammatory response syndrome (SIRS) and the compensatory anti-inflammatory response syndrome (CARS) [4]. These changes acting in concert disrupt immune regulation and the resulting imbalance between the two arms predisposes the injured patient to opportunistic infections and trauma related complications at timepoints where the imbalance is greatest.
It is now generally agreed that tissue destruction and necrotic cell death caused by trauma initiates the early host response to traumatic injury. This results in the systemic release of previously compartmentalized cellular antigens and factors [5][6]. These endogenous mediators are called alarmins because they alert the immune system to tissue damage and are part of a category of damage associated molecular patterns (DAMPs). Furthermore, pathogen associated molecular patterns (PAMPs) initiate anti-microbial immune responses to combat opportunistic pathogens when the injured tissues liberate their microbiome or the injured person is exposed to nosocomial infections [7–9]. Many different types of DAMPs and PAMPs have been described and some use shared signaling receptor pathways. Subsequent to recognizing DAMPs and PAMPs by cells, a myriad of signaling molecules transmit messages to regulate immune responses to trauma and infections. However, inflammatory cytokine production may be exaggerated in injured people that develop opportunistic infections, which makes patient management more difficult and can lead to septic shock and other clinical complications. Therefore, a better understanding of circulating DAMPs, PAMPs and signaling molecules in trauma that alter immune cells and response phenotypes may be useful in the diagnosis of trauma-induced immune dysregulation, prediction of mortality, and clinical management of severely-injured people.
Few studies have focused on the hyper-acute effects of trauma plasma on normal PBMC as a surrogate for the injured patient’s in vivo milieu. Previous studies have relied primarily on the direct analysis of immune cells from injured patients [10–12]. In the present study, we harnessed an exploratory approach to underpin the significance of trauma-induced systemic factors on altering immune cell subsets and evaluate the key variables influencing these changes. We show that culturing peripheral blood mononuclear cells (PBMCs) from healthy, un-injured people with plasma from trauma patients alters immune cell subsets and by this approach we identified specific changes in natural killer (NK) cells and CD8+ T cells.
Methods
Trauma Patient Selection:
Patients were enrolled from May to October 2015 from Brigham and Women’s Hospital (BWH) who met the following inclusion criteria: over the age of 18 years, not pregnant, with Injury Severity Score (ISS) greater than 20, no medical history, or medications predisposing immune dysregulation (e.g., chemotherapy or steroid use). ISS was estimated at admission, and final calculation was performed after discharge. The trauma patient plasma samples used in this study were from 5 male patients with an average age of 25.2 ± 6.2 years over two timepoints, day 1 and 3. Blood was drawn into Vacutainer EDTA Tubes (BD, Franklin Lakes, NJ) at days 1 and 3 after injury. Blood samples were also collected from healthy, uninjured, age- and gender-matched volunteers. This clinical study protocol was approved by the Partner’s Institutional Review Board (IRB).
Plasma Preparation:
Blood samples were aliquoted into a centrifugation tube and diluted 1:1 by addition of PBS. Sepmate tubes (STEM CELL Technologies Inc, Vancouver, Canada) were used to isolate PBMCs by density centrifugation by adding 4.5mL of Ficoll (GE healthcare Ficoll-Plus). The diluted blood was overlaid onto the Ficoll. Samples were centrifuged at 1200 × g for 10 minutes, plasma was removed and frozen at −80°C in aliquots.
Peripheral Blood Mononuclear Cell (PBMC) Isolation:
Blood (4ml) from normal individuals was aliquoted into a tube and 4mL PBS was added. Sepmate tubes were prepared by adding 4.5mL of Ficoll to each tube. The diluted blood was overlaid onto the Ficoll and tube were centrifuged at 1200 × g for 10 minutes. Following this, the Sepmate tube was quickly inverted into a fresh 15mL tube to harvest the PBMCs. Culture medium was added to the samples and centrifuged at 200 × g for 5 minutes to pellet PBMCs. PBMCs were frozen at 20 × 106 cells per ml using Cryostor CS10 freezing medium (BioLife Solutions, Bothell, WA) using Nalgene Mr. Frosty containers following the manufacturers’ protocol.
Plasma and PBMC Co-Culture:
Normal PBMCs were thawed at 37°C for 3 minutes and then mixed into 37°C thawing medium consisting of RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum, 1 mM glutamine, 10 mM HEPES, 2 mM nonessential amino acids, 2.5 × 10−5 M 2-mercaptoethanol, 20 units/mL penicillin/streptomycin/amphotericin B, 20 units/ml sodium heparin and 25 units/mL benzonase nuclease (both from Sigma-Aldrich, St. Louis, MO). Cells were then centrifuged at 100 × g for 10 minutes after which they were incubated in 10mL XVIVO™ 15 media (Lonza, Walkersville, MD, USA) for 30 minutes at 37°C to recover. PBMCs (1 × 106) were added to individual wells of a 96-well tissue culture treated round-bottom polystyrene plate (Corning Life Sciences, Tewksbury MA, USA). To each well, 5% plasma (n=5 for all plasma groups; control, 1 day post trauma (trauma-1d) and 3 days’ post trauma (trauma-3d)) was added to cells for a final volume of 200μL. PBMCs and plasma samples were cultured for 24 hours at 37°C.
To test the effects of DAMPS and bacterial lipopolysaccharide (LPS) on PBMC responses, PBMCs from 3 different donors were cultured with CPG DNA sequence 2336 (CpG2336) (3μg/mL), ND6 formyl peptide (100nM), homogenized human liver (liver DAMPs, 100μg/mL), ATP (5mM) or E. coli LPS (100 ng/mL) in X-VIVO™ 15 media for 24 hours. Cells were collected at the end of the timepoint as described above and stained for CyTOF analysis.
CyTOF Staining and Data Analysis:
Following a 24-hour culture period, PBMCs were transferred to a new 96-well round-bottom polypropylene plate and centrifuged at 750 × g for 3 minutes. During this centrifugation, warm 2mM EDTA-PBS was added to the culture wells to detach all adherent cells. The detached and suspension cells were combined and washed once by centrifugation. After washing cells, cisplatin (0.1 mM) viability staining reagent (Fluidigm, South San Francisco, CA) was added for 2 minutes. After centrifugation, TruStain FcX Fc receptor blocking reagent (BioLegend) was added for 10 minutes in CyTOF staining buffer (CSB; calcium/magnesium-free PBS, 0.2% BSA and 0.05% sodium azide). CyTOF antibodies were labeled in-house using the MaxPar kit (Fluidigm). The CyTOF antibody panel is shown in the Supplemental Table. All CyTOF staining was performed at room temperature and all washes were performed by centrifugation of staining plates or tubes at 750 × g for 3 minutes. Cell surface staining antibodies in CSB were added directly to samples and incubated for 30 minutes followed by 2 washes with CSB. Cells were fixed and permeabilized using the FoxP3 Staining Buffer Set (Thermo Fisher Scientific, Waltham, Massachusetts, USA). After centrifugation, cells were barcoded using palladium barcoding reagents and subsequently combined into a single sample for pooled intracellular antibody staining [13]. After 30 minutes, the cells were washed and then fixed with 1.6% PFA. To stain DNA, 18.75 μM iridium intercalator solution (Fluidigm) was added to the cells. Cells were washed in Cell Acquisition Solution (Fluidigm), EQ four element calibration beads (Fluidigm) were added, and then samples were acquired on a Helios CyTOF Mass Cytometer (Fluidigm).
CyTOF data was cleaned to remove debris and subsequently normalized, debarcoded, and uploaded into Cytobank (Mountain View, CA) for analysis. Data was gated to generate live, normalization bead negative single cells by eliminating debris and doublets. Following this, major immune cell populations were identified by manual gating strategies and a sample gating strategy is provided in supplemental figure 1 (Fig. S1). viSNE maps were generated by equal single-cell event sampling set at the lowest number of cells per sample in the experiment. Following subsampling, viSNE analysis was run for 2000 iterations at a perplexity of 50 and random seed to organize the data into 2D space based on marker expression similarity, which were then used for downstream analysis. For SPADE analysis, target node number was determined by k-nearest neighbor cluster by RPhenoGraph and the agglomerative clustering was performed on live cells on t-SNE1 and t-SNE2 parameters.
Luminex multiplex cytokine detection assay:
For multiplex cytokine analysis of plasma, samples were collected and frozen directly at −80°C. The NET MFI of cytokines were determined in control plasma and trauma patient plasma samples from 1- and 3-days post injury using Luminex technology. The assay was conducted using 20μL of plasma sample and cytokines were determined by standard curve analysis. The 30 cytokine panel included; TNFa, IL-18, IL-1a, IL-1b, IL-1RA, IL-10, IL-33, IL-23, IL-22, IL-6, IL-21, IL-8, Tweak, MCP-1, IFNy, G-CSF, MIP-1a, GM-CSF, Trem-1, GRO alpha, ENA-78, IL-17A, PDGF-AA, PDGF-BB, MCP-3, MIG, MDC, IP-10, IL-15 and Flt3L. The plate was read and analyzed on a Luminex FLEXMAP 3D instrument (Luminex Corporation, Austin, Texas).
Statistical analysis:
CyTOF data were analyzed using Cytobank (Mountain View, CA). Gating was performed and event count and expression data of cell populations was exported. All statistical analysis was performed in GraphPad Prism (La Jolla, CA).
Results
viSNE map of human peripheral blood mononuclear cells
To gain a global overview of how plasma from healthy people and trauma patients affect human peripheral blood mononuclear cell (PBMC) numbers and phenotypes, we cultured healthy PBMC with 5% plasma (healthy, trauma-1d or trauma-3d) for 24 hours and stained samples with a CyTOF panel containing metal-tagged antibodies to detect changes in surface and intracellular markers on major immune cell subsets. CyTOF staining data from these samples were normalized, compensated, and subsequently equal sampled for all downstream analysis. Immune cells were gated in live singlets and concatenated by group (healthy, trauma-1d or trauma-3d) for viSNE map generation.
When applied to this dataset, viSNE generated a two-dimensional map in space that separated different immune cell subsets based on marker expression (Fig. 1A). Importantly, this analysis accurately reduced the high-dimensional CyTOF data into plots that clustered CD4+ T cells, CD8+T cells, B cells, NK cells, and myeloid populations based on marker expression profiles (Fig. 1B). We observed that trauma plasma did not change detection profiles of these subset markers on PBMCs as compared to normal plasma.
Figure 1. PBMC staining and viSNE map generation.
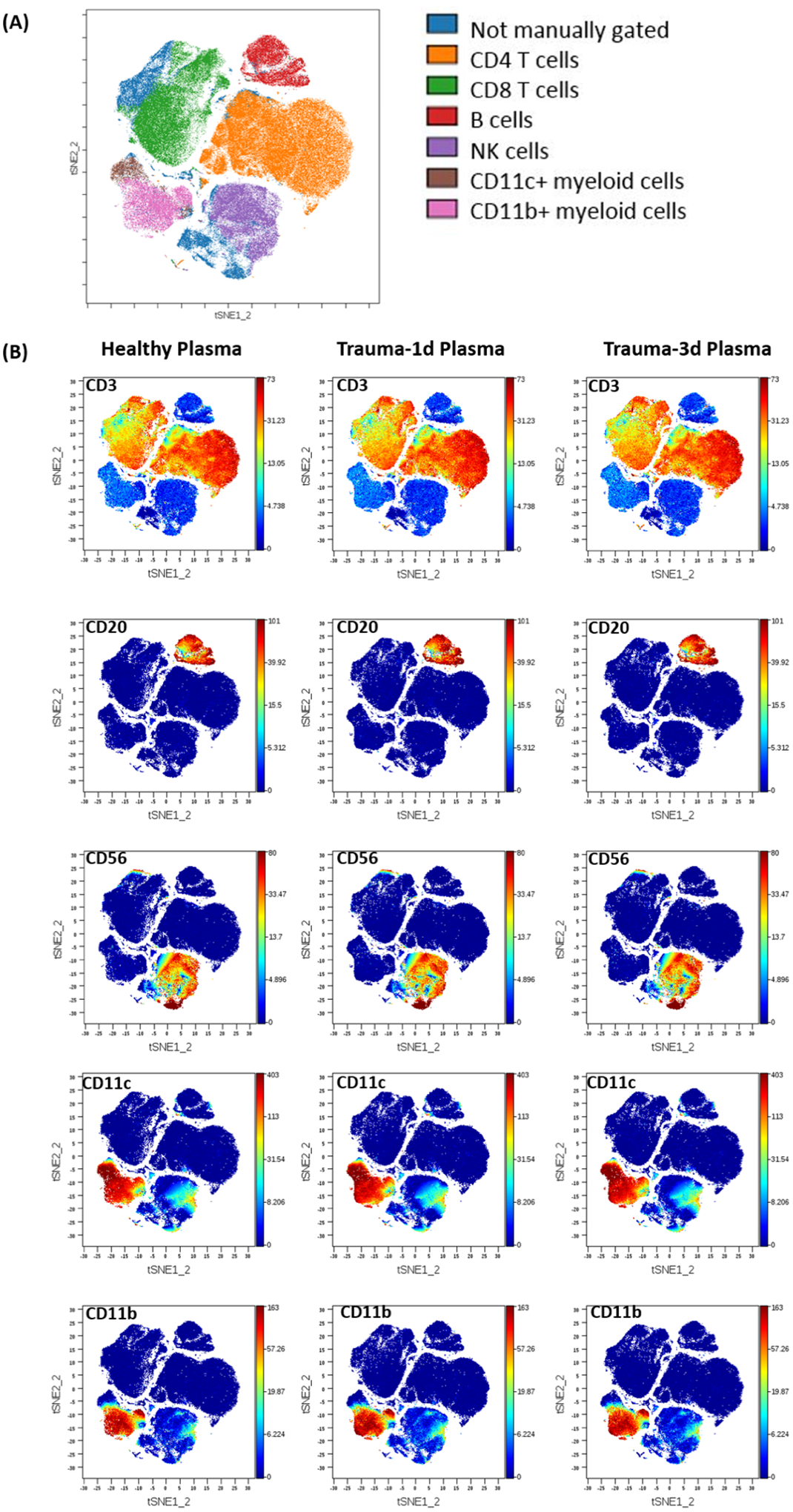
PBMCs from healthy donors were stained using surface and intracellular markers of immune cell lineages by our panel (Supplemental Table) for a total measurement of 29 parameters. Intact single cells were gated based on Ir-191 DNA stains. Viable cells were selected based on negativity for cisplatin staining. All FCS files were first normalized using control beads and compensated by CATALYST using calibration beads mixed with individual CyTOF antibodies. Following this, the files were analyzed using Cytobank, web-based software system. (A) Cell populations defined by manual gating strategies were projected onto t-SNE space and assigned specific colors. (B) Immune cell subsets were identified and manually gated based on the signal intensity of the phenotypic markers. Trauma-1d is plasma taken from injured patients 1 day after injury, trauma-3d is plasma taken from injured patients 3 days after injury.
Trauma plasma induces an increase in CD8+ T cell and Natural Killer cell abundance, but a reduction of CD4+ T cells.
Traumatic injuries induce a complex multicellular immune response that can lead to a subsequent imbalance in immune system homeostasis. To gain new insights into systemic mediators of trauma-induced changes in immune cell compartments, we established an experimental approach to measure the effects of plasma from trauma patients on normal human PBMC activation profiles. The present study employed a qualitative approach that involved culturing PBMCs from 4 individual normal healthy donors with 5% plasma (n= 5 per group; healthy, trauma-1d or trauma-3d) for 24 hours to investigate the hyper-acute effects of trauma plasma and circulating factors on different immune cell abundances by CyTOF mass cytometry.
The cell abundance counts of T cell subsets, B cells and natural killer cells (NKs) were quantified and compared between groups (Fig. 2). As shown, there was a significant decrease in total CD3+ CD4+ T cells when cultured with trauma-1d plasma but not trauma-3d plasma in comparison to healthy plasma (Fig. 2A). There was no statistical difference in CD20+ B cell counts between healthy and trauma plasma groups (Fig. 2B). In marked contrast, CD3+ CD8+ T cells and NK cells showed significant increases in abundance when cultured with plasma that was prepared from patients 1 day after injury (Figs. 2C–D).
Figure 2. Trauma plasma induces immune cell subset changes.
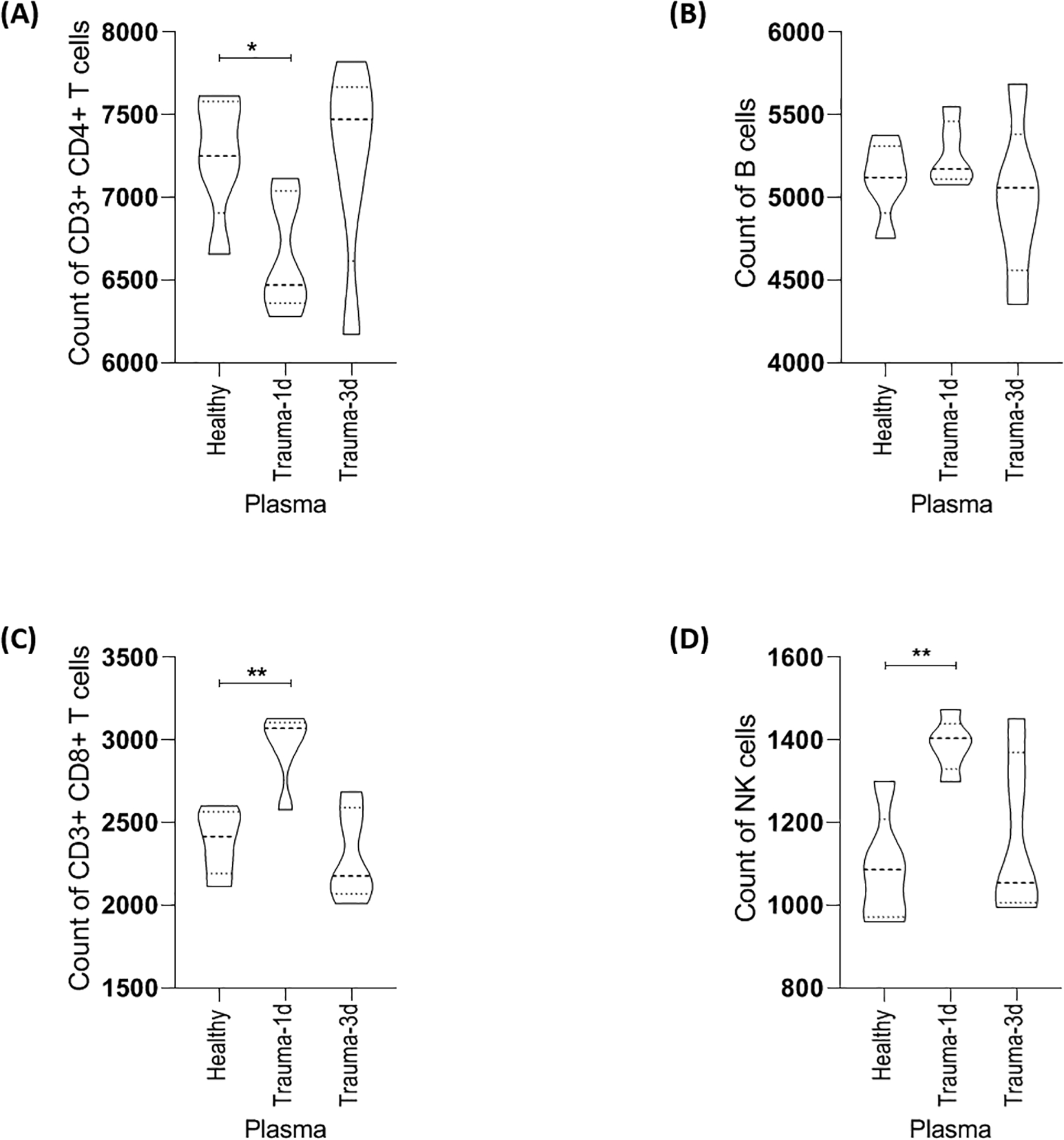
CyTOF staining data on immune cell subsets from PBMC donors cultured with 5% plasma (n=5 plasma samples in healthy, trauma-1d and trauma-3d groups) were randomly equal sampled for analysis and a representative donor is displayed here. (A) Culture of healthy PBMCs with plasma taken 1 day after injury, but not 3 days after injury, caused a significant decrease in the count of CD3+ CD4+ T cells. (B) Trauma plasma did not significantly alter the number of CD3- CD20+ B cells in comparison to healthy plasma. (C) & (D) Plasma taken 1 day after injury, but not 3 days after injury, caused a significant increase in the count of CD3+ CD8+ T cells and CD3- CD56+ CD16+ NK cells, respectively. Data is representative of 4 PBMC donors. Healthy; healthy plasma. Trauma-1d; plasma harvested from patients 1 day after injury. Trauma-3d; plasma harvested from injured patients 3 days after injury. Unpaired t-tests were used for statistical analysis. *p ≤ 0.05, **p ≤ 0.01.
Distinct CD4+ T cells are affected by trauma plasma.
CD4+ T cells are key players in the production of cytokines that provide activating signals to innate cells, resulting in the initiation of antimicrobial effector activity; a response pivotal to controlling susceptibility to trauma-associated infections. In this study, we found the addition of trauma-1d plasma to PBMC cultures reduces the overall count of CD3+ CD4+ T cells, which prompted a deeper phenotypical examination of this cell population. To do this, we examined density plots and observed a heterogeneous distribution of CD3+ CD4+ T cells that were clustered into 2 subpopulations labelled as A and B (Fig. 3A). Interestingly, we found that the density of subpopulation B was increased in PBMCs cultured with trauma-1d plasma, and to a lesser extent, with trauma-3d plasma (Fig. 3A). To better characterize this cell population, we performed phenotypical analyses on both subpopulations. This analysis demonstrated that population B is characterized by expression of CD38 and CD197 (CCR7) (Fig. 3B). To validate this finding, we gated this population and observed a significant increase in the percentage of CD38+ CD197+ cells in CD3+ CD4+ T cells in PBMCs that were cultured with trauma plasma (Fig. 3C).
Figure 3. Identification of T cell subsets altered by trauma plasma.
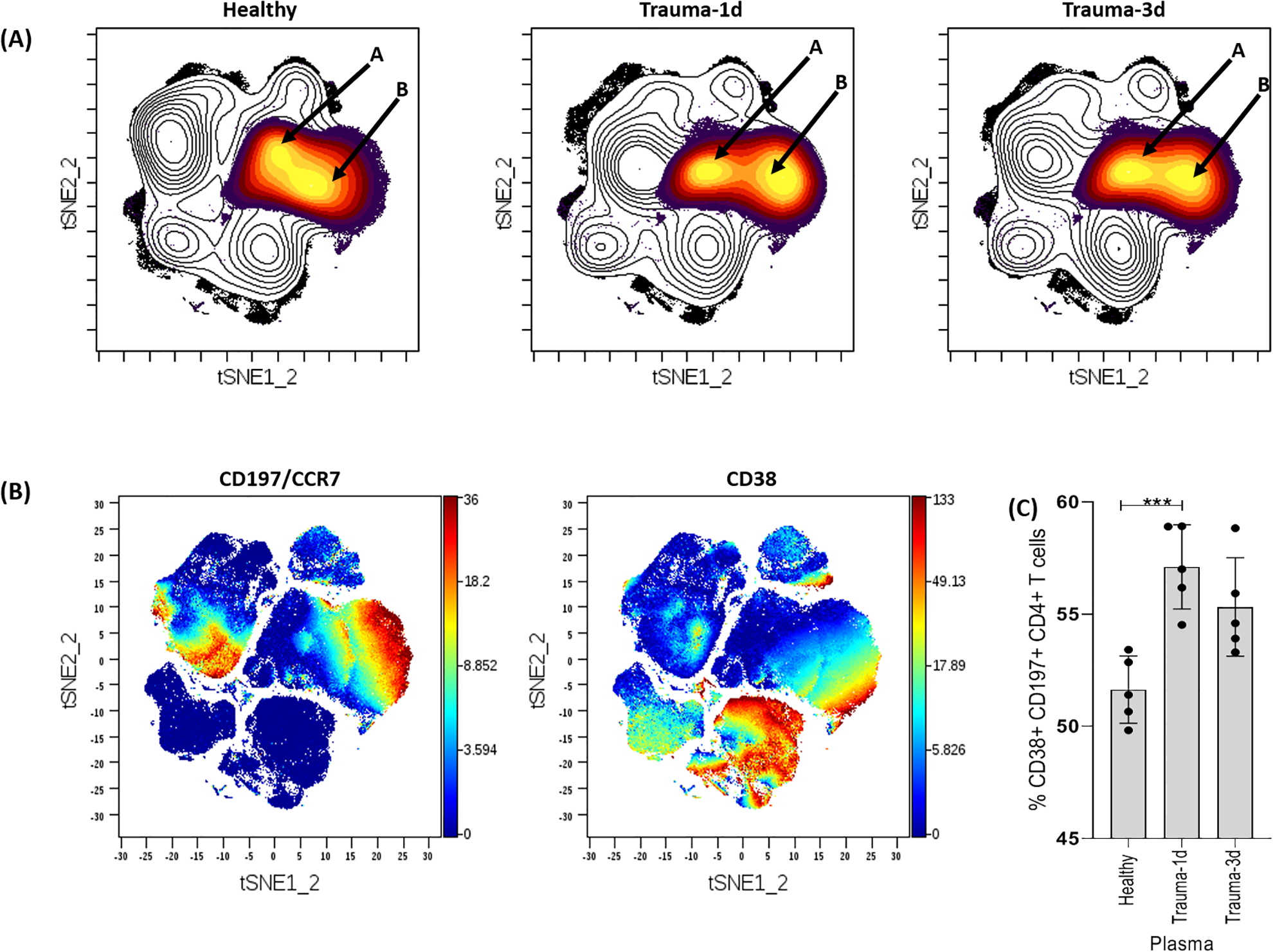
(A) Cells were identified by viSNE and T cells were identified based on CD3 expression. The density plot revealed 2 major subpopulations of CD3+ CD4+ T cells designated as A and B (see arrows). The density of population B within CD3+ CD4+ T cells was increased when cultured with trauma-1d plasma compared to the healthy plasma group. (B) The populations were identified by viSNE automatic clustering and separated based on CD197 (CCR7) and CD38 expression. (C) The frequency of T cell subset B was significantly higher in CD3+ CD4+ T cells cultured with trauma-1d plasma. Error bars are standard error of the mean (SEM) and unpaired t-tests were used for statistical analysis. Data representative of 4 PBMC donors cultured with 5% plasma (n=5 plasma samples per group; healthy plasma, trauma-1d plasma or trauma-3d plasma) and equal sampled for analysis. ***p ≤ 0.001.
CD11c+ Natural Killer Cells are increased by trauma plasma.
Natural killer cells were found to expand in PBMCs cultured with trauma-1d plasma in comparison to healthy plasma. To confirm our findings, we used several different analytical approaches to validate and better characterize these cells. First, our data was submitted to the Cytofkit R package to determine cluster numbers in our CyTOF dataset by PhenoGraph, which use k-means and density-based clustering to estimate the number of different cell clusters in the dataset. We then performed unsupervised Spanning-tree Progression Analysis of Density-normalized Events (SPADE) analysis on tSNE1 and tSNE2 parameters. This approach organized the cell phenotyping data into a tree-based representation of the cell populations.
By this computational approach, we were able to identify separate clusters of NK cells, CD4+ and CD8+ T cells, B cells, and CD11b+ or CD11c+ myeloid cells (Fig. 4A). Next, we wanted to see if our previous finding of increased NK cells by trauma-1d plasma in manually-gated samples was replicated in this unsupervised analysis. As shown in Figure 4B, the number of NK cells (SPADE identified cluster 16) were significantly increased when cultured with trauma-1d plasma but not trauma-3d plasma thus confirming our previous findings. To fully characterize the NK cells identified by the unsupervised SPADE clustering program, we exported the mean marker expression data for cluster 16. Interestingly, we found that these NK cells were highly positive for CD11c, a type-I transmembrane protein classically expressed in monocytes and macrophages (Fig. S2). We sought to confirm alignment of the SPADE-identified NK cells with our previous findings by manual gating. First, we exported NK cell cluster 16 (orange cluster) and overlaid it onto our previously generated viSNE plot (blue background) (Fig. 4C). Next, we wanted to confirm that the cells in that overlaid area of the viSNE plot were NK cells. As shown in Figure 4C, the viSNE plots illustrate the colocalization of NK cell markers CD56, CD16, T-bet and CD11c with cluster 16. Lastly, given that CD11c and CD38 were SPADE-identified markers on NK cells that we had not gated, we employed a reverse manual biaxial gating strategy on SPADE-identified markers on our data to examine how this manually-gated population abundance aligns with the unsupervised SPADE results. We confirmed that trauma-1d plasma increased the abundance of this unique NK cell population when manually gated on SPADE-identified markers (Fig. 4D). Thus, we combined unsupervised analysis and manual biaxial analytical methods to discover and validate that plasma from day 1 trauma patients significantly induces the activation and increase in a population of CD11c+ NK cells from normal PBMCs.
Figure 4. Spanning-tree Progression Analysis of Density-normalized Events (SPADE) analysis identifies trauma plasma induced alterations of NK numbers.
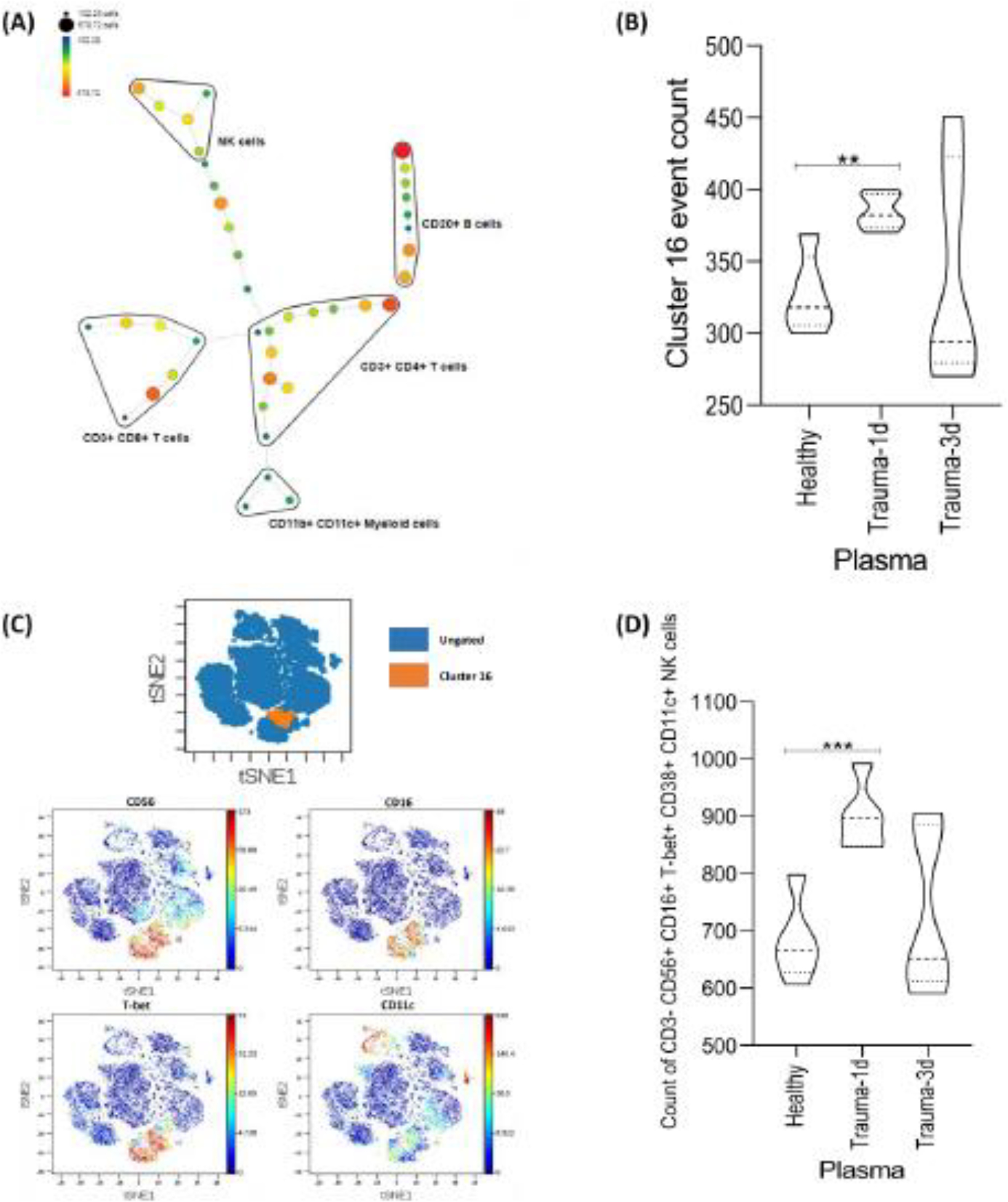
(A) SPADE tree overview of immune cell subsets. Nodes are colored by count. (B) Clusters were exported to a new experiment and the event count data was analyzed. SPADE identified a significant increase in the abundance of NK cells when cultured with trauma-1d plasma, confirming our findings by viSNE map analysis. (C) Cluster 16 was exported and overlaid onto viSNE plots to confirm alignment with manually defined NK cells. (D) A manual, biaxial gating strategy was applied to equal sampled data using markers that were identified by SPADE (cluster 16) to confirm alignment of supervised and unsupervised NK cells. Data representative of 4 PBMC donors cultured with 5% plasma (n=5 plasma samples per group) and an unpaired t-test was used for statistical analysis between healthy and each trauma group. **p ≤ 0.01, *** p ≤ 0.001.
Trauma plasma increases CD73+ CCR7+ CD8+ T cells.
CD8+ T cells are important mediators of cytotoxic adaptive immunity and we found that trauma-1d plasma increased this subpopulation in PBMC cultures by manual gating. We used the same unbiased, deep phenotyping approach that we used for NK cells to further characterize these cells and confirm alignment of our supervised and unsupervised analysis. This analysis strategy revealed an increase in CD8+ T cells that co-express CD73 and CCR7, defined as cluster 14 by SPADE (Fig. 5A). Furthermore, when we manually gated on CD3+ CD8+ CD73+ CCR7+ T cells, we found an increase in this subpopulation of cells when cultured with trauma-1d plasma but not trauma-3d plasma hence, verifying the unsupervised SPADE-identified CD8+ T cells (Fig. 5B).
Figure 5. SPADE analysis identifies trauma plasma induced alterations of CD8+ T cells.
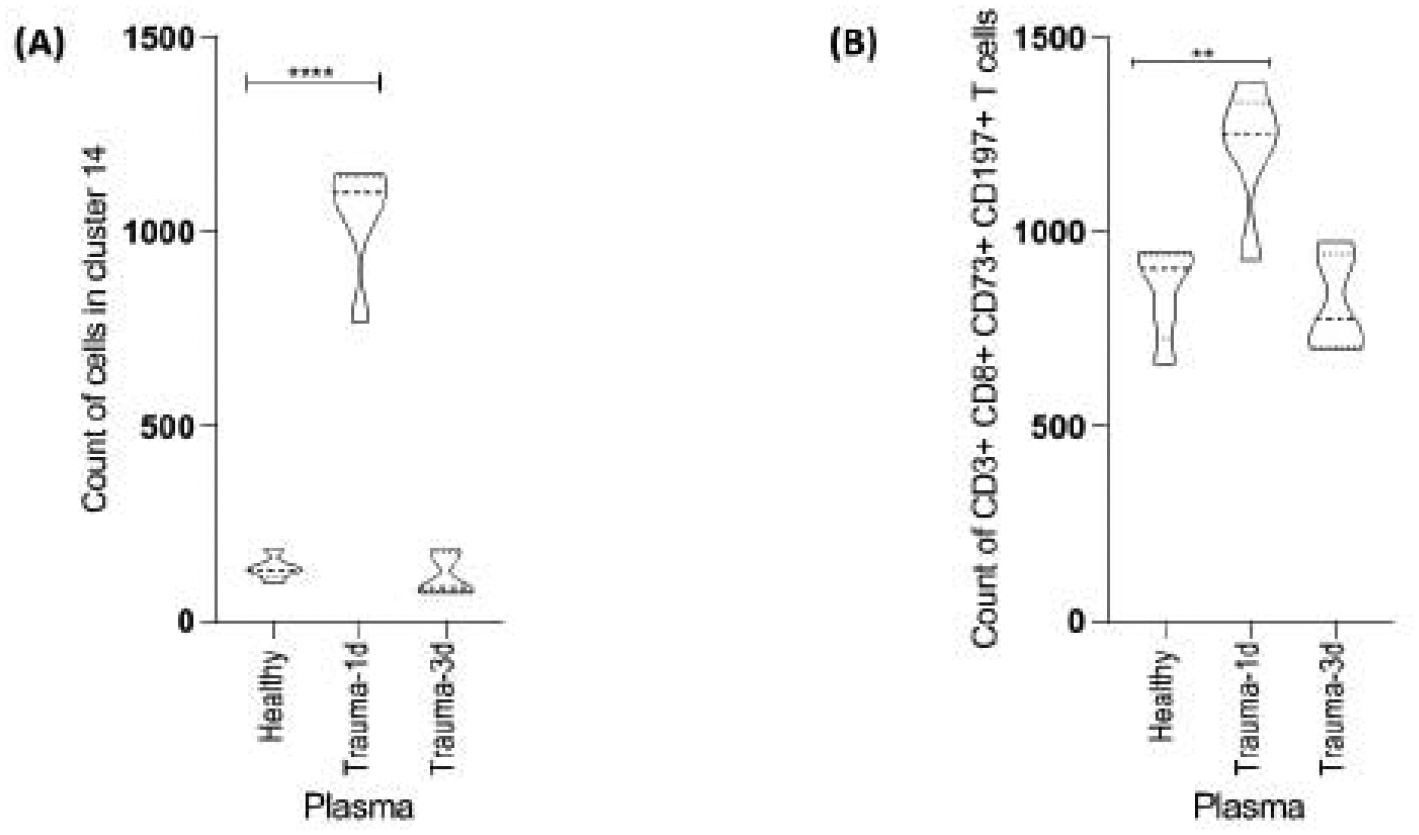
(A) Computational analysis of samples clustered by SPADE identified an increase in a population of CD3+ CD8+ T cells (CD3+ CD8+ CD73+ CCR7+ cells in cluster 14), confirming previous findings by viSNE and manual gating. (B) A manual, biaxial gating strategy was applied to equal sampled data using markers that were identified by SPADE (cluster 14) to confirm alignment of supervised and unsupervised CD3+ CD8+ T cells. Data representative of 4 PBMC donors cultured with 5% plasma (n=5 plasma samples per group; healthy plasma, trauma-1d plasma or trauma-3d plasma) and an unpaired t-test was used for statistical analysis between healthy and each trauma group. **p ≤ 0.01, **** p ≤ 0.0001.
Multiplex analysis of circulating cytokines and growth factors shows differential regulation and heightened inflammatory signatures of trauma patients, and culture of PBMC with DAMPS and LPS increases CD11c+ NK cells.
Traumatic injuries trigger the production and secretion of cytokines and cell signaling molecules via multiple pathways. Our data shows that trauma plasma, particularly trauma-1d plasma, quantitively and significantly increased NK cells and CD8+ T cells by CyTOF mass cytometry analysis. Here, we conducted screening analysis using Luminex multiplex technology to examine the trauma-induced cytokine and growth factor signature of the plasma samples used in our study as an attempt to explore circulatory mechanisms responsible for controlling NK cell and CD8+ T cell increase in abundance.
Plasma prepared from trauma-1d and trauma-3d patients in addition to the age-matched and sex-matched healthy controls used previously were screened for cytokines, growth factors and chemokines by Luminex multiplex technology to determine changes in plasma profiles of injured people. The Net MFI values of select markers are shown in figure 6. Here, we demonstrate that among the analytes we measured, IL-1RA and macrophage-derived chemokine (MDC) were notably increased by trauma. Furthermore, IL-6, Granulocyte colony-stimulating factor (G-CSF) and IL-15 were statistically significantly higher in trauma-1d patient plasma compared to healthy controls. In contrast, levels of platelet-derived growth factor (PDGF)- AA and PDGF-BB were progressively decreased by trauma, with PDGF-AA significantly reduced at 3 days after injury. There was a slight increase in Net MFI values of FLT3L in trauma-3d plasma, but this was not significantly induced. There were no significant differences in the levels of IL-10 and IL-21 despite a gradual reduction by trauma.
Figure 6. Plasma cytokine expression is altered by trauma.
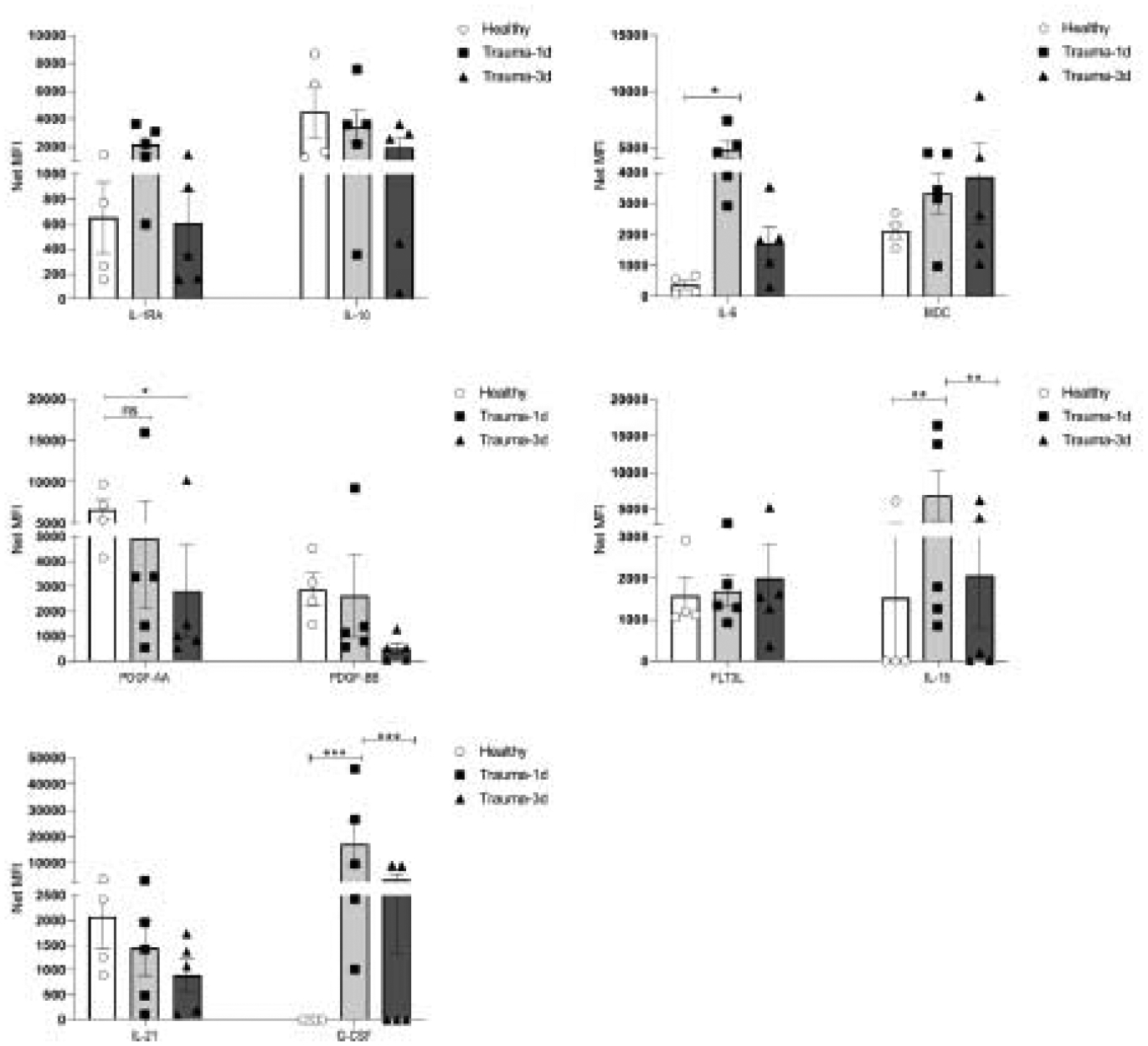
Plasma prepared from trauma-1d and trauma-3d patients in addition to age-matched and sex-matched healthy controls used previously were screened by Luminex multiplex cytokine detection technology to assess changes in cytokine levels and profiles in trauma patients. Cytokines showing differences between healthy control plasma and trauma patients are shown here from a panel of 30 cytokines. The quantification of analytes is reported as NET MFI for comparisons. N= 5 for trauma plasma groups and n=4 for healthy controls. Data is representative of 3 independent experiments and a two-way ANOVA was used for statistical analysis. * p ≤0.05, **p ≤ 0.01, *** p ≤ 0.001.
To support our hypothesis that circulating DAMPS released by trauma and present in the plasma increased the abundance of CD11c+ NK cells, we cultured three individual PBMC donors with mitochondrial DAMPS (mtDAMPs) or LPS for 24 hours. We observed an increase in fold change of CD11C+ NKs when cultured with CPG DNA (CPG2336), the mitochondrial formyl peptide – ND6, liver DAMPs and LPS (Fig. S3). The observed effects of these DAMPs on NK cell activation suggest that mitochondrial CpG DNA and formylated mitochondrial proteins may be important mediators of NK cell activation and abundance in trauma.
Discussion
The trauma immunology field has expanded significantly since in the 1990s coincident with advances in our understanding of immunology and the realization that traumatic injuries can profoundly alter immune functions and phenotypes. Since then, a large amount of research has been carried out to understand the mechanisms underlying trauma-related complications with the aim to improve clinical management of trauma. However, despite these advances, accidental injury is still the leading cause of death among people aged 1 to 44 years old. In addition, the development of post-trauma complications like poor anti-microbial immune function, heightened systemic inflammatory syndromes, development of sepsis, and chronic critical illness remain large clinical problems. To advance understanding of the trauma immune reaction, we performed this study to identify human immune cell subsets that react to factors that are systemically released following trauma. To accomplish this, we used CyTOF mass cytometry as a method to allow us to profile dynamic phenotypic changes in peripheral blood lymphocyte subsets when cultured with plasma from healthy volunteers versus trauma patients. The data here clearly show that trauma-induced systemic factors modulate distinct changes in conventional CD3+ T cell subsets and natural killer cells. We show the utility of this new approach to explore and evaluate the acute effects of trauma plasma on these normal immune cell subsets as a surrogate for the trauma microenvironment.
Modulation of circulating lymphocyte abundances in trauma patients is a well-described observation [11,14,15]. By comparing trauma and normal plasma additions to PBMCs as a model system, we demonstrate that there are circulating factors that significantly change or alter T cells and NK cells. Populations of CD8+ T cells and NK cells were expanded in vitro by day 1 trauma plasma, while CD4+ T cells were reduced. Interestingly, these changes happened in response to day 1 trauma plasma, but not plasma from day 3 trauma patients. This finding highlights the acute and time-dependent nature of trauma induced circulating factors that modulate human lymphoid cells. Deep analysis of CyTOF staining data revealed the presence of a distinct CD4+ T cell subpopulation characterized by expression of CCR7 and CD38 that uniquely increased in frequency in CD4+ T cells that reacted to trauma plasma. This is an important finding because CCR7 is responsible for the homing and recruitment of leukocytes to lymphoid organs where they can participate in the initiation of an immune response by readily proliferating and differentiating into effector cells [16]. The expression of CCR7 indicates that these cells could be either naïve or central memory cells but given that we did not have CD45RA or CD45RO markers present in our CyTOF panel, we cannot definitively identify them as one or the other. However, the expression of CD38 on these cells would suggest that they were activated by trauma plasma [17][18]. The implications of these findings are that trauma plasma may partially recapitulate an in vivo traumatic injury, whereby CD4+ T cells (naïve or central memory) are activated and then migrate to lymphoid tissues to interact with antigen presenting cells (APCs).
To validate our finding that trauma-1d plasma increased NK cell count by manual gating strategies, we employed an unbiased, unsupervised analysis approach to confirm the alignment of our cell populations identified by manual and non-manual gating techniques. When the data was subjected to SPADE analysis, the changes in NK cell abundance by trauma-1d and −3d plasma was validated. When we further explored the phenotype of NK cells identified by SPADE, we found that they were positive for CD11c. This is interesting because CD11c, an integrin, is typically known as a marker of myeloid cells and not conventionally known to be expressed on NK cells. In addition to facilitating binding of the cell cytoskeleton to the extracellular matrix, CD11c also binds to complement iC3b to trigger phagocytosis[19] [20]. Therefore, a possible role for increased numbers of CD11c+ NKs in trauma is that they participate in controlling infection through the recognition of opsonized pathogens or DAMPs. This may be beneficial for an injured patient who needs to be ready to resist opportunistic nosocomial infections. In a murine model of muscle traumatic injury, an increase in the percentage and absolute number of NK cells was reported in the lymph nodes of injured mice when compared to the sham group; this increase was associated with decreased Th1-type immune responses [21]. In contrast, a study performed in a murine polytrauma mouse model suggested that NK cells contributed to the pathogenesis of polytrauma and worsened responses to bacterial infection [22]. Thus, different types of traumatic injuries may have distinct impacts on NK cells activation and biology.
Here, we identified a unique CD11c+ trauma reactive NK cell. Data on human CD11c+ NK cells are lacking. Therefore, in this context, we believe that establishing parallels between murine and human data is prudent. A study by Burt et. al. revealed that CD11c identified a distinct NK subpopulation in the murine liver that displayed enhanced lytic capability and antigen presenting cell (APC) function [23]. Moreover, it was suggested that murine CD11c+ NK cells possess a bitypic phenotype given the expression of CD11c and moderate APC function, and have in some cases been termed interferon-producing killer DC (IKDC) [24]. Studies to better characterize these cell subsets in humans are needed. Interestingly, one report using human CD34+ cells stimulated by dialyzable leukocyte extracts (DLE) showed an increase in CD11c+ NK cells from CD34+ cells that were capable of enhanced IFNγ production and inducing γδ T cells [25]. DLE is composed of disrupted PBMCs and thus represent a mixture of DAMPs and proteins, akin to trauma plasma and therefore provides theoretical support for our findings.
There is a wealth of data on the impact of trauma and complications such as sepsis on CD8+ T cells, with many studies showing a sepsis-induced CD8+ T cell lymphodepletion and impaired function [26–29]. Data concerning an increase in CD8+ T cells in trauma is not as well-described but may reflect that CD8+ T cells leave the circulation in response to infections or injuries. One study demonstrated a robust increase in total CD8+ T cell number in the skin-draining lymph nodes of mice subjected to a full-thickness burn [30]. Moreover, CD8+ T cell numbers from the wound site were increased in burn-injured mice compared to sham 24 hours after injury [31]. These studies suggest that CD8+ T cells may be increased only at the wound site or injury-site draining lymph nodes which may not be the case in severe sepsis. Our data showed an increase in CD73+ CCR7+ CD8+ T cells. The expression of CD73 on these cells may serve as a co-activator and immunosuppressive ectoenzyme to generate adenosine from extracellular ATP [32] and given that the cells were increased with trauma-1d plasma, but not trauma-3d plasma, suggests that the cells are acutely activated by systemic factors in trauma at earlier timepoints.
The production of cytokines is critical for anti-microbial immunity in injured patients. However, trauma may also lead to the exaggerated production of these signaling proteins, which may contribute to uncontrolled inflammation and tissue damage. Our data provide convincing evidence of a link between pro-inflammatory cytokines in the trauma-1d plasma samples and the increased NK cell and CD8+ T cell numbers at that timepoint. The early elevation of IL-6 by trauma and sepsis is well-described, thus it is not surprising that we found a significant increase in IL-6 in trauma-1d plasma but not trauma-3d plasma. Trauma plasma also had increased levels of GM-CSF and although its role in trauma is not as well-documented as IL-6, it has pro-inflammatory functions and stimulates the bone marrow to increase myeloid cell hematopoiesis [33]. Trauma-1d plasma had significantly high IL-15 levels. IL-15 is the major cytokine involved in NK cell development and differentiation and also activates CD8+ T cells [34–37]. The importance of IL-15 in the immune system has been highlighted by IL-15 knock-out mice that demonstrate reduced numbers and maturation of immune cells, namely NK cells and CD8+ T cells [38,39]. Interestingly, a study by Aranami et. al. determined that in humans, CD11c expression by NK cells was induced by IL-15 in addition to several inflammatory cytokines but that this subpopulation may negatively participate in the immune regulation of multiple sclerosis [40]. Therefore, it seems possible that the increase in CD11c+ NK cells and CD8+ T cells in this study may predominantly be due to the increase in IL-15. A recent study administered IL-15 superagonist to burn-injured, infected mice and found no survival advantage conferred by this treatment despite alterations of a number of immune cell subsets [41]. Future studies will need to focus on the IL-15, NK cells, and CD8+ T cell axis to provide new insights into how trauma activates and regulates the immune system.
Lastly, we wanted to explore the effects of DAMPs on NK cell activation. In the current study, CPG-DNA, mitochondrial ND6 formyl peptide, liver DAMPs, and LPS increased CD11c+ NK cell counts after a 24-hour co-culture. Interestingly, CD11c+ NK cells were more reactive than their CD11c- counterparts. Intriguingly, a study by Pillarisetty et. al. reported that murine NK cells possessing the bitypic phenotype of NK1.1 and CD11c respond to CpG-DNA and expand in vivo [42]. Therefore, it is logical that circulating DAMPs may also be responsible for the trauma-1d plasma induced increase in CD11c+ NK cells.
There are limitations with this study. First, we acknowledge that our dataset is composed of relatively small group sizes. 4 normal PBMC donors were each cultured with healthy (n=5), trauma-1d (n=5) and trauma-3d (n=5) plasma samples for a total of 15 plasma samples per PBMC donor. However, despite the relatively small patient cohort, the data significantly demonstrates the power of our approach to examine the effect of trauma plasma on human immune cells that led to the identification of trauma plasma reactive CD8+ T cells and CD11c+ NK cells. Second, when we analyzed PBMCs cultured with mtDAMPs and LPS, we observed a variation between the PBMC donors. This is not surprising since human PBMC donor variation is normal. Nevertheless, we were able to use this novel approach to identify NK cells and CD8+ T cells as immune cell types that respond acutely to systemic factors in trauma plasma.
Taken together, our data provides the first demonstration that using 5% trauma plasma as a surrogate for the trauma microenvironment can modulate T cell subsets and a unique NK cell subpopulation. Furthermore, we show that trauma-1d plasma, but not trauma-3d plasma, display increased levels of inflammatory cytokines that may contribute to these alterations. We show that this approach provides a rational method to identify immune cell subsets that react to traumatic injury in humans. Thus, this study contributes to the existing literature concerning the activation and induction of T cell subsets and NK cells in the early timepoints following traumatic injury and supports the importance of investigating the early interplay between cytokines, DAMPs and PAMPs in trauma immunology.
Supplementary Material
Highlights.
Early trauma plasma increases CD11c+ natural killer cells.
CD8+ T cells are increased by circulating factors in trauma plasma.
Increased pro-inflammatory cytokines in trauma plasma are associated with at an earlier timepoint.
Financial Support for the Study:
T32 GM 103702-5 and Department of Defense grant # W81XWH-16-1-0464
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
References:
- [1].(WHO) W H O. The global burden of disease: 2004 update. 2008.
- [2].Centers for Disease Control and Prevention 2017. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) Nonfatal Injury Data.
- [3].Centers for Disease Control and Prevention 2017. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) Fatal Injury Data.
- [4].Stoecklein VM, Osuka A and Lederer JA 2012. Trauma equals danger--damage control by the immune system J. Leukoc. Biol 92 539–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. , 2005. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion J. Exp. Med 201 1135–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang Q, Itagaki K and Hauser CJ 2010. Mitochondrial DNA is released by shock and activates neutrophils via P38 map kinase Shock 34 55–9 [DOI] [PubMed] [Google Scholar]
- [7].Tsan MF and Gao B 2007. Review: Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors J. Endotoxin Res 13 6–14 [DOI] [PubMed] [Google Scholar]
- [8].Lai Y, Nardo A Di, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. , 2009. Commensal bacteria regulate TLR3-dependent inflammation following skin injury Nat. Med 15 1377–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hua F, Wang J, Ishrat T, Wei W, Atif F, Sayeed I et al. , 2011. Genomic profile of Toll-like receptor pathways in traumatically brain-injured mice: Effect of exogenous progesterone J. Neuroinflammation 8 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fabian TC, Croce MA, Fabian MJ, Trenthem LL, Yockey JM, Boscarino R et al. , 1995. Reduced tumor necrosis factor production in endotoxin-spiked whole blood after trauma: Experimental results and clinical correlation Surgery 118 63–72 [DOI] [PubMed] [Google Scholar]
- [11].Seshadri A, Brat GA, Yorkgitis BK, Keegan J, Dolan J, Salim A, et al. , 2017. Phenotyping the Immune Response to Trauma: A Multiparametric Systems Immunology Approach Crit. Care Med 45 1523–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kirchhoff C, Biberthaler P, Mutschler WE, Faist E, Jochum M and Zedler S 2009. Early down-regulation of the pro-inflammatory potential of monocytes is correlated to organ dysfunction in patients after severe multiple injury: A cohort study Crit. Care 13 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zunder ER, Finck R, Behbehani GK, Amir EAD, Krishnaswamy S, Gonzalez VD, et al. , 2015. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm Nat. Protoc 10 316–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Organ BC, Antonacci AC, Chiao J, Chiao J, Kumar A, De Riesthal HF, et al. , 1989. Changes in lymphocyte number and phenotype in seven lymphoid compartments after thermal injury Ann. Surg 210 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gupta DL, Bhoi S, Mohan T, Galwnkar S and Rao DN 2016. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis Cytokine 88 214–21 [DOI] [PubMed] [Google Scholar]
- [16].Sallusto F, Geginat J and Lanzavecchia A 2004. C entral M emory and E ffector M emory T C ell S ubsets : Function, Generation, and Maintenance Annu. Rev. Immunol 22 745–63 [DOI] [PubMed] [Google Scholar]
- [17].Delia D, Zaccolo M, Malavasi F, Funaro ADA, Giulio C, Roggero S and Malavas F 1997. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. Why The JI ? Submit online. • Rapid Reviews ! 30 days * from submission to initial decision • No Triage ! Every submission reviewed by practicing sci Immunity 7 315–24 [Google Scholar]
- [18].Gossez M, Rimmelé T, Andrieu T, Debord S, Bayle F, Malcus C, et al. , 2018. Proof of concept study of mass cytometry in septic shock patients reveals novel immune alterations Sci. Rep 8 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sandor N, Lukacsi S, Ungai-Salanki R, Orgovan N, Szabo B, Horvath R, et al. , 2016. CD11c/CD18 dominates adhesion of human monocytes, macrophages and dendritic cells over CD11b/CD18 PLoS One 11 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bilsland CA, Diamond MS and Springer TA 1994. The leukocyte integrin p150,95 (CD11c/CD18) as a receptor for iC3b. Activation by a heterologous beta subunit and localization of a ligand recognition site to the I domain. J. Immunol 152 4582–9 [PubMed] [Google Scholar]
- [21].Wirsdorfer F, Bangen MJ, Pastille E, Hansen W and Flohe BS 2015. Breaking the cooperation between bystander T-cells and natural killer cells prevents the development of immunosuppression after traumatic skeletal muscle injury in mice Clin. Sci 128 825–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Barkhausen T, Frerker C, Pu C, Pape H, Krettek C and Van Griensven M 2008. Depletion of NK cells in a murine polytrauma model is associated with improved outcome and a modulation of the inflammatory response Shock 30 401–10 [DOI] [PubMed] [Google Scholar]
- [23].Burt BM, Plitas G, Stableford JA, Nguyen HM, Bamboat ZM, Pillarisetty V G a et al. , 2008. CD11c identifies a subset of murine liver natural killer cells that responds to adenoviral hepatitis J. Leukoc. Biol 84 1039–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].GeurtsvanKessel CH, Bergen IM, Muskens F, Boon L, Hoogsteden HC, Osterhaus ADME, et al. , 2009. Both conventional and interferon killer dendritic cells have antigen-presenting capacity during influenza virus infection PLoS One 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ramírez-Ramírez D, Vadillo E, Arriaga-Pizano LA, Mayani H, Estrada-Parra S, Velasco-Velázquez MA, et al. , 2016. Early Differentiation of Human CD11c + NK Cells with γδ T Cell Activation Properties Is Promoted by Dialyzable Leukocyte Extracts J. Immunol. Res 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yves Le Tulzo, Céline Pangault, Arnaud Gacouin, Valérie Guilloux OT, Laurence Amiot, Pierre Tattevin, et al. , 2002. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome Shock 18 487–94 [DOI] [PubMed] [Google Scholar]
- [27].Condotta SA, Rai D, James BR, Griffith TS and Badovinac VP 2013. Sustained and Incomplete Recovery of Naive CD8 + T Cell Precursors after Sepsis Contributes to Impaired CD8 + T Cell Responses to Infection J. Immunol 190 1991–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sharma A, Yang W-L, Matsuo S and Wang P 2015. Differential alterations of tissue T-cell subsets after sepsis Immunol Lett. 168 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Choi YJ, Kim SB, Kim JH, Park SH, Park MS, Kim JM, et al. , 2017. Impaired polyfunctionality of CD8+ T cells in severe sepsis patients with human cytomegalovirus reactivation Exp. Mol. Med 49 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Valvis SM, Waithman J, Wood FM, Fear MW and Fear VS 2015. The Immune Response to Skin Trauma Is Dependent on the Etiology of Injury in a Mouse Model of Burn and Excision J. Invest. Dermatol 135 2119–28 [DOI] [PubMed] [Google Scholar]
- [31].Rani M and Schwacha MG 2017. The composition of T-cell subsets are altered in the burn wound early after injury PLoS One 12 4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dianzani U, Redoglia V, Bragardo M, Attisano C, Bianchi A, Di Franco D, et al. , 1993. Co-stimulatory signal delivered by CD73 molecule to human CD45RAhiCD45ROlo (naive) CD8+ T lymphocytes. J. Immunol 151 3961–70 [PubMed] [Google Scholar]
- [33].Hamilton JA and Anderson GP 2004. GM-CSF biology Growth Factors 22 225–31 [DOI] [PubMed] [Google Scholar]
- [34].Ohteki T, Ho S, Suzuki H, Mak TW and Ohashi PS 1997. Role for IL-15/IL-15 receptor beta-chain in natural killer 1.1+ T cell receptor-alpha beta+ cell development. J. Immunol 159 5931–5 [PubMed] [Google Scholar]
- [35].Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, et al. , 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice J. Exp. Med 191 771–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ranson T, Vosshenrich CAJ, Corcuff E, Richard O, Müller W and Di Santo JP 2003. IL-15 is an essential mediator of peripheral NK-cell homeostasis Blood 101 4887–93 [DOI] [PubMed] [Google Scholar]
- [37].Steel JC, Waldmann TA and Morris JC 2012. Interleukin-15 biology and its therapeutic implications in cancer Trends Pharmacol. Sci 33 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S et al. , 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation Immunity 9 669–76 [DOI] [PubMed] [Google Scholar]
- [39].Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. , 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice J. Exp. Med 191 771–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Aranami T, Miyake S and Yamamura T 2006. Differential Expression of CD11c by Peripheral Blood NK Cells Reflects Temporal Activity of Multiple Sclerosis J. Immunol 177 5659–67 [DOI] [PubMed] [Google Scholar]
- [41].Patil NK, Luan L, Bohannon JK, Guo Y, Hernandez A, Fensterheim B and Sherwood ER 2016. IL-15 superagonist expands mCD8+T, NK and NKT cells after burn injury but fails to improve outcome during burn wound infection PLoS One 11 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pillarisetty VG, Katz SC, Bleier JI, Shah AB and DeMatteo RP 2005. Natural Killer Dendritic Cells Have Both Antigen Presenting and Lytic Function and in Response to CpG Produce IFN-γ via Autocrine IL-12 J. Immunol 174 2612–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


