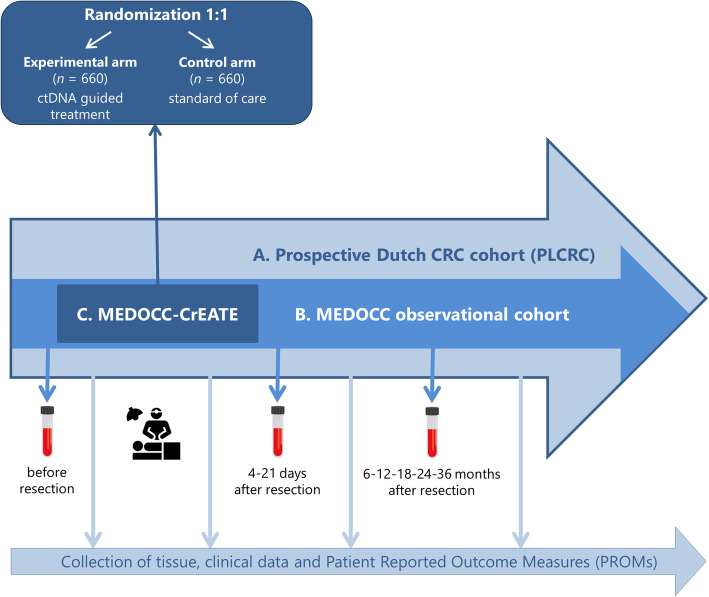
Fig. 1.
Schematic presentation of MEDOCC-CrEATE, using the trial within cohort (TwiCs) design. a PLCRC is a nationwide cohort study in the Netherlands with inclusion of CRC patients (all stages). By optional informed consent regarding collection of biomaterials and future randomization, observational as well as interventional trials can be performed within the cohort. b Non-metastatic CRC patients are included in MEDOCC when the patient signs informed consent for PLCRC including additional blood sampling. Blood samples are withdrawn before resection, 4–21 days after resection and every 6 months during the first 3 years of follow-up. c Eligible stage II colon cancer patients are randomized 1:1 following the TwiCs design. In the experimental group informed consent is being asked for immediate ctDNA analysis of the blood sample obtained after resection. If ctDNA is detectable, patients are offered adjuvant chemotherapy. The control group is not informed about MEDOCC-CrEATE and will receive standard of care