Abstract
Purpose
Neurological manifestations of COVID-19 infection include impaired consciousness, strokes, and seizures. Limited reports describing EEG abnormalities in patients with COVID-19 have been published. These articles reported nonspecific encephalopathic patterns, epileptiform discharges, and rarely seizures. Our primary aim was to assess EEG abnormalities in patients with COVID-19 and evaluate for epileptiform activity or seizures.
Methods
We identified five critically ill adult patients with COVID-19 who underwent EEG monitoring. All patients had Ceribell™ rapid response EEG initially and two continued with conventional long-term video EEG.
Results
All 5 patients had encephalopathy and 3 also had seizure-like movements, thus prompting EEG monitoring. EEGs all showed nonspecific markers of encephalopathy including diffuse slowing and generalized rhythmic delta activity. Two also had epileptiform discharges reaching 2−3 Hz at times, with one patient in nonconvulsive status epilepticus and the other developing clinical status epilepticus with myoclonic movements. EEG and clinical symptoms improved with anti-seizure medications.
Conclusion
Status epilepticus was present in 2 out of our cohort of 5 critically ill patients who underwent EEG monitoring. These findings highlight the importance of EEG monitoring in high-risk patients with COVID-19 and encephalopathy. EEG recordings in such patients can identify pathological patterns that will benefit from treatment with anti-seizure medications.
Keywords: Case series, COVID-19, EEG, Seizures, Encephalopathy
1. Introduction
COVID-19, an acronym for “coronavirus disease of 2019,” is a disease caused by the pathogen “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2) [1]. This illness can have neurologic manifestations including stroke, impaired consciousness, skeletal muscle injury, anosmia or dysgeusia, headache, dizziness, and seizures [2].
Patients with COVID-19 infection can have clinical seizures and nonconvulsive seizures without overt motor symptoms causing altered mentation. Due to the risk of exposure to electroencephalography (EEG) technicians, conventional EEG monitoring is typically reserved for situations where the suspicion for seizures is high. An alternative is Ceribell™ rapid response EEG (rapid-EEG) (Ceribell Inc., Mountain View, CA, USA), which can be quickly applied by healthcare personnel without prior EEG technology training. Its use enables screening for seizures in high-risk patients [3] and reduces the risk of exposure to healthcare personnel.
Data on EEG findings of patients with COVID-19 still remain scarce. In a recent case series, epileptiform discharges were seen in 40.9% of COVID-19 positive patients with frontal sharp waves as the predominant pattern [4]. Several other case studies showed that patients can have diffuse slowing, bifrontal discharges, or generalized periodic discharges [5,6]. There were rare reports of status epilepticus confirmed on EEG recordings [[7], [8], [9], [10], [11]].
In our case series, we report the findings of five critically ill patients with COVID-19 infection who underwent EEG monitoring.
2. Methods
In this retrospective case series, we identified 5 adult patients admitted either at Stanford Hospital or Stanford ValleyCare Hospital between June 1st and July 4th, 2020 who were critically ill from COVID-19 infection and underwent EEG monitoring. Informed consent was obtained from the patients’ family. They were all in the intensive care unit (ICU), requiring intubation and compassionate use remdesivir. The patients were diagnosed with COVID‐19 by SARS-CoV-2 RNA detection from nasopharyngeal swab testing using FDA-approved assays. They all had nonlesional head computed tomography (CT) except for one patient. EEGs were typically ordered for mental status changes or seizure-like activity. All patients had rapid-EEG initially and two subsequently transitioned to conventional long-term video EEG monitoring.
Rapid-EEG recordings used a 10-electrode (8-channel) system, consisting of Fp1, Fp2, F7, F8, T3, T4, T5, T6, O1, and O2, and read in a reduced double banana montage. Sampling rate was 250 Hz and high and low pass filters were 1 Hz and 70 Hz respectively. Conventional continuous video EEG was acquired with a digital EEG machine (Neurofax EEG-1200, Nihon Kohden Co., Tokyo, Japan) and included 24 channels with the international 10–20 system for electrode placements and an EKG lead. Sampling rate was 500 Hz, with a time constant of 0.1 s (high pass filter of ∼1.59 Hz) and low pass filter of 70 Hz. The gain was set at 50 μV for rapid-EEG and 7 μV for conventional EEG, with a notch filter of 60 Hz. Rapid-EEG was placed by nursing staff and conventional EEG was applied by EEG technicians. All EEG recordings were reviewed by an epilepsy fellow and a supervising board-certified epileptologist.
3. Results
3.1. Patient 1
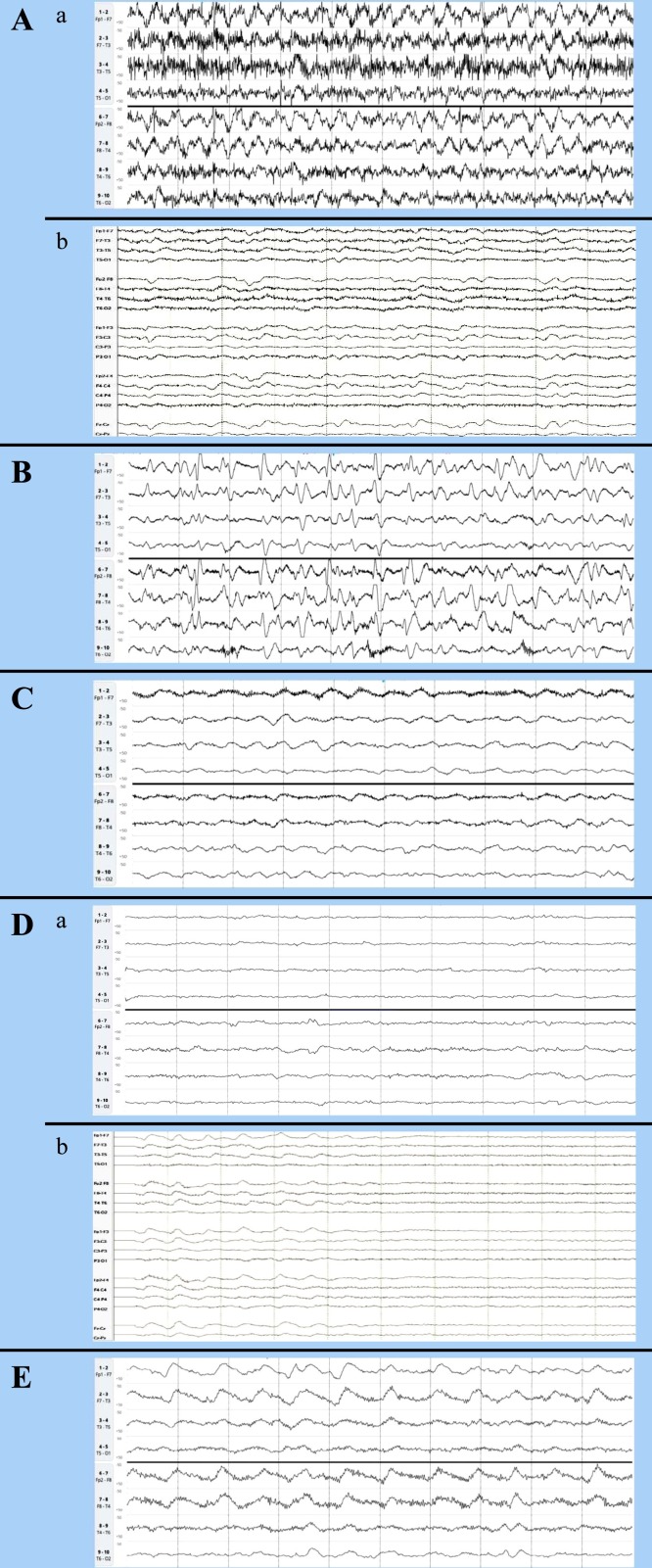
A 37-year-old female with multiple comorbidities including end-stage renal disease on dialysis presented with 1 week of diarrhea. Initial COVID-19 testing was negative but repeat testing one day later was positive. She was intubated and sedated on midazolam infusion. Two days later, she had increased oxygen requirements, worsening mental status, and full body jerking movements. Rapid-EEG showed continuous spike and slow wave discharges that appeared bifrontal predominant at up to 3 Hz associated with myoclonic movements, consistent with status epilepticus (Fig. 1 A-a). She was loaded with levetiracetam and later phenytoin and started on maintenance levetiracetam and phenytoin. Her myoclonic movements resolved and her EEG concurrently showed less frequent discharges. Given ongoing encephalopathy, she had two lumbar punctures (LP) performed which were unremarkable. She later also had conventional continuous EEG, which showed diffuse slowing with intermittent generalized rhythmic delta activity (GRDA) and occasional generalized periodic discharges (GPDs) at 0.5−1 Hz (Fig. 1A-b). Discharges slowly improved the course of the recordings, as well as her mental status.
Fig. 1.
EEG recordings in the 5 patients with COVID-19 infection viewed in 10 s epochs. Rapid-EEG recordings are in a limited double banana montage viewed at a sensitivity of 50 μV, with the top 4 channels representing the left hemisphere and the bottom 4 channels representing the right hemisphere. Conventional EEG recordings are in double banana montage viewed at a sensitivity of 7 μV.
(A) a. Initial rapid-EEG on the first patient showing 2−3 Hz bifrontal predominant spike and wave discharges.
b. Conventional EEG showing improvement after starting on anti-seizure medications, with generalized rhythmic delta activity (GRDA) and occasional generalized periodic discharges (GPDs) at mostly 0.5−1 Hz.
(B) Rapid-EEG on the second patient showing GPDs reaching 2−3 Hz at times.
(C) Rapid-EEG on the third patient showing moderate-severe diffuse slowing and GRDA.
(D) a. Initial rapid-EEG on the fourth patient showing severe diffuse slowing.
b. Conventional EEG performed later showing moderate-severe diffuse slowing and GRDA.
(E) Rapid-EEG on the fifth patient showing moderate diffuse slowing and GRDA.
3.2. Patient 2
A 60-year-old female with hypertension presented with progressive shortness of breath, myalgias, diarrhea, and chest and abdominal pain for 1 week. She was found to have COVID-19 and subsequently intubated and sedated with midazolam. Rapid-EEG was ordered for encephalopathy, which showed abundant 1 Hz GPDs with occasional bursts at 2−3 Hz (Fig. 1B), concerning for nonconvulsive status epilepticus. Around the time of the EEG recording, she also had hypernatremia, a left peroneal deep vein thrombosis, bleeding from a chest wall hematoma, and left apical pneumothorax. An LP was not performed for encephalopathy. She was started on levetiracetam with improvement in her discharges and mentation and later recordings showing moderate diffuse slowing and GRDA.
3.3. Patient 3
A 50-year-old male without significant medical history was brought in for 1 day of shortness of breath and myalgias and found to be COVID-19 positive. He also had an episode of whole body shaking prior to arrival. He was intubated and sedated on propofol. LP performed was unremarkable. He was loaded with levetiracetam and started on maintenance therapy. Rapid-EEG was placed and showed moderate to severe diffuse slowing and intermittent GRDA (Fig. 1C).
3.4. Patient 4
A 38-year-old female with complex comorbidities including orthotopic heart transplant and heterotopic kidney transplant, pulmonary hypertension, diabetes, and chronic congestive hepatopathy was brought in after being found down by family with seizure-like movements. Initial COVID-19 testing in the emergency room was negative but was later positive when retested a few days later due to worsening encephalopathy and fevers. She was intubated and sedated on propofol. Patient was connected to rapid-EEG and started on levetiracetam. EEG showed mostly severe diffuse slowing with a low amplitude background (Fig. 1D-a). There were no epileptiform discharges or seizures. Due to ongoing encephalopathy and the desire for prolonged EEG recording, rapid-EEG was converted to conventional EEG, which showed moderate-severe diffuse slowing with intermittent GRDA (Fig. 1D-b). She also had an LP, which was unremarkable.
3.5. Patient 5
A 40-year-old male with atrial fibrillation, hypertension, and diabetes with multiple vascular complications including chronic kidney disease presented with fevers, generalized weakness, nausea, and vomiting. He was found to have COVID-19 infection and in hypoxic respiratory failure. He was subsequently intubated and sedated on dexmedetomidine. LP was unremarkable. CT head showed multifocal hypodensities, which were suspected to be small subacute-chronic strokes due to atrial fibrillation. These lesions would not explain his acute encephalopathy, so he received rapid-EEG which showed moderate diffuse slowing and GRDA (Fig. 1E).
4. Discussion
We describe five critically ill patients with COVID-19 who underwent EEG monitoring due to altered mentation or seizure-like movements. The EEGs in all the patients showed some degree of diffuse slowing and GRDA, both nonspecific markers of encephalopathy. Two patients also had epileptiform discharges that reached >2.5 Hz, meeting criteria for status epilepticus [12]. One patient had myoclonic movements correlating with the discharges. The discharges improved in both patients with the administration of anti-seizure medications; the patient with myoclonic activity also had resolution of her movements.
Like prior reports, this case series demonstrates that EEG abnormalities in COVID-19 positive patients consist of nonspecific indicators of encephalopathy and epileptiform activity including bifrontal predominant and generalized discharges. However, a limited montage EEG was used in most patients and might not be able to detect focal findings in the parasagittal region.
In our cohort, we also found that 2 out of 5 patients had ictal patterns on their EEGs meeting criteria for status epilepticus initially. These patterns and their clinical symptoms subsequently improved with anti-seizure medications. This case series demonstrates that there is potentially a pathogenic role of COVID-19 infection in triggering de novo seizures and status epilepticus in patients, highlighting the importance of EEG monitoring in patients with altered mentation or seizure-like activity. EEG recordings in such patients can identify pathological patterns that will benefit from treatment with anti-seizure medications. However, the patients in our cohort were all critically ill and most had underlying chronic medical problems including chronic kidney disease, making them more susceptible to nonspecific EEG abnormalities as well as status epilepticus. Patient 2 also had multiple complications during her ICU stay, further increasing her risk of seizures. The clinical benefit and risk of exposure from EEG evaluation need to be considered. Rapid-EEG may be a more efficient way of screening patients for seizures and status epilepticus and limit the risk of exposure. Further studies elucidating the epileptogenesis of COVID-19 infection are needed.
Funding
This paper did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We would like to acknowledge the EEG technicians and nursing staff who placed the EEGs on our patients, the fellows and attendings who participated in reviewing the EEGs, all the staff members who provided excellent care to the patients, and our patients from whom we have learned so much and who made this paper possible.
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westover M.B., Gururangan K., Markert M.S., Blond B.N., Lai S., Benard S., et al. Diagnostic value of electroencephalography with ten electrodes in critically ill patients. Neurocrit Care. 2020 doi: 10.1007/s12028-019-00911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galanopoulou A.S., Ferastraoaru V., Correa D.J., Cherian K., Duberstein S., Gursky J., et al. EEG findings in acutely ill patients investigated for SARS‐CoV2/COVID‐19: a small case series preliminary report. Epilepsia Open. 2020;5(2):314–324. doi: 10.1002/epi4.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vespignani H., Colas D., Lavin B.S., Soufflet C., Maillard L., Pourcher V., et al. Report of EEG finding on critically ill patients with COVID‐19. Ann Neurol. 2020 doi: 10.1002/ana.25814. June 13 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flamand M., Perron A., Buron Y., Szurhaj W. Pay more attention to EEG in COVID-19 pandemic. Clin Neurophysiol. 2020;131(8):2062–2064. doi: 10.1016/j.clinph.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balloy G., Mahé P.J., Leclair-Visonneau L., Péréon Y., Derkinderen P., Magot A., et al. Non-lesional status epilepticus in a patient with coronavirus disease 2019. Clin Neurophysiol. 2020;131(8):2059–2061. doi: 10.1016/j.clinph.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hepburn M., Mullaguri N., George P., Hantus S., Punia V., Bhimraj A., et al. Acute symptomatic seizures in critically Ill patients with COVID-19: is there an association? Neurocrit Care. 2020 doi: 10.1007/s12028-020-01006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somani S., Pati S., Gaston T., Chitlangia A., Agnihotri S. De novo status epilepticus in patients with COVID‐19. Ann Clin Transl Neurol. 2020;7(7):1240–1244. doi: 10.1002/acn3.51071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vollono C., Rollo E., Romozzi M., Frisullo G., Servidei S., Borghetti A., et al. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020;78:109–112. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beniczky S., Hirsch L.J., Kaplan P.W., Pressler R., Bauer G., Aurlien H., et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54:28–29. doi: 10.1111/epi.12270. [DOI] [PubMed] [Google Scholar]