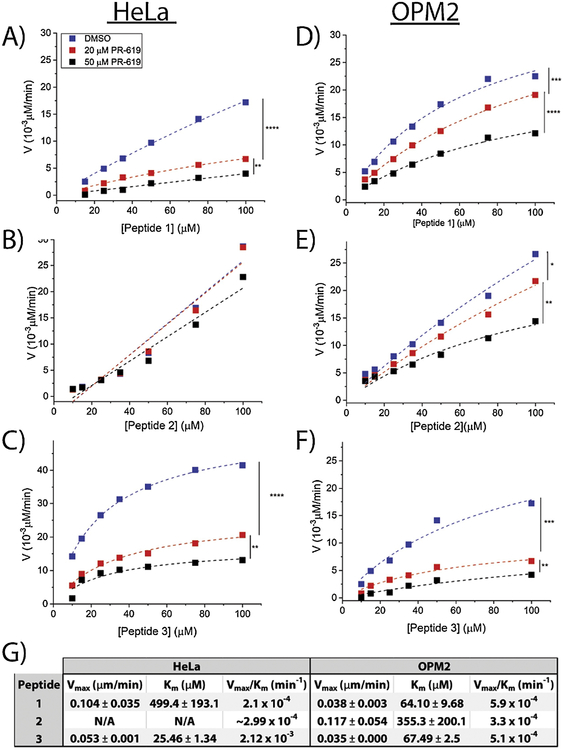
Figure 2. Kinetics of DUB-mediated hydrolysis of peptides in HeLa and OPM2 lysates.
Concentration-dependent DUB-mediated cleavage rates of Peptide 1 (A), Peptide 2 (B), and Peptide 3 (C) in HeLa lysates (4 mg/mL) and Peptide 1 (D) Peptide 2 (E), and Peptide 3 (F) in OPM2 lysates (4 mg/mL). Lysates were pre-treated with 20 μM PR-619, 50 μM PR-619, or DMSO. Legends are the same in all plots. Rate data demonstrate Michaelis-Menten kinetics (r2>0.99) in all cases. (G) Michaelis-Menten kinetics parameters calculated for the DMSO curves. All peptides efficiently distinguish samples treated with varying concentrations of the inhibitor ([I]=0, 20, 50 μM) corresponding to the specificity of the peptides to DUBs. ‘****’ denotes p<10−10, ‘***’ denotes p<10−5, ‘**’ denotes p<10−4, ‘*’ denotes p<0.005.