Extended Data Fig. 1 |. Comparison between flow cytometry and CFUs for L. monocytogenes infection.
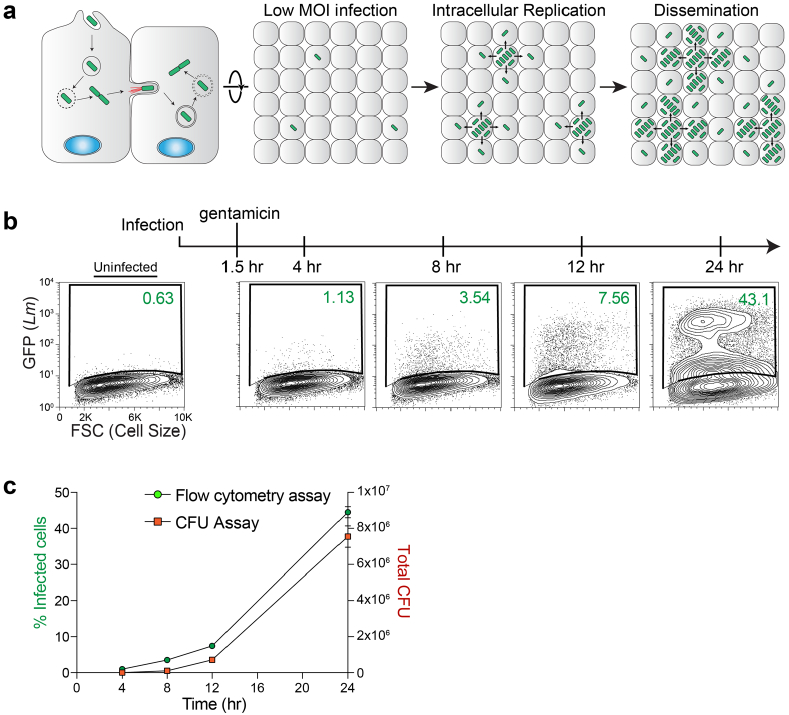
a, Schematic of the L. monocytogenes life-cycle (left) and its replication and intercellular dissemination initiated from a low dose of bacterial infection. HEK293A cells were infected with GFP-expressing L. monocytogenes (MOI=1) so that only a small percentage of the host cell monolayer (<1%) are initially infected. Cell-to-cell spread of L. monocytogenes results in robust infection of the monolayer over time. b, Representative flow cytometry plots of L. monocytogenes (GFP) infection of HEK293A cells at the indicated time points. After 90 minutes of infection, the host cell monolayers were washed and incubated with gentamicin to remove and kill extracellular bacteria. These studies were repeated independently four times with similar results. c, Direct comparison between gentamicin protection assays assessed by flow cytometry (as above) or Colony forming Units (CFUs). Samples were harvested for analysis at the indicated time points after infection. Graph showing the percent of infected cells determined by flow cytometry (y-axis, left) were directly compared to CFUs recovered (y-axis, right). Mean values from 4 independent experiments are plotted, and error bars show s.d. We concluded that flow cytometry is an accurate method of enumerating bacterial burden in host cells.
