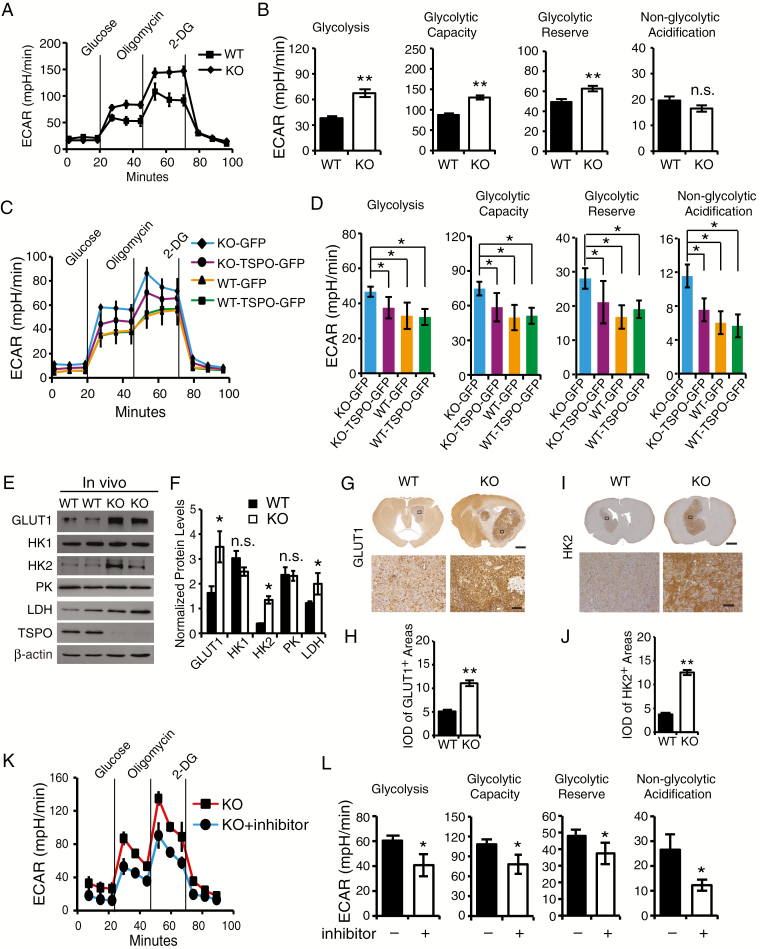
Fig. 4.
TSPO deficiency enhances glycolysis in GL261 cells and gliomas. (A) The glycolytic stress test to measure glycolytic activities in TSPO-KO and WT GL261 cells. (B) Quantification of glycolysis, glycolytic capacity, glycolytic reserve and nonglycolytic acidification in (A). (C) Measurement of glycolytic activities in TSPO-KO and WT GL261 cells transfected with TSPO-GFP or GFP expression constructs. (D) Quantification of glycolytic activities in (C). (E) Western blot analysis of the levels of GLUT1, HK1, HK2, PK, and LDH in TSPO-KO and WT gliomas. (F) Normalized levels of the proteins shown in (E). (G) Representative images of immunohistochemical staining with an anti-GLUT1 antibody to examine GLUT1 expression in TSPO-KO and WT gliomas. Scale bar, 1 mm for images in the upper panels and 100 μm for images in the lower panels. (H) Quantification of GLUT1 expression in TSPO-KO or WT gliomas shown in (G). (I) Representative images of immunohistochemical staining with an anti-HK2 antibody to examine HK2 expression in TSPO-KO and WT gliomas. Scale bar, 1 mm for images in the upper panels and 100 μm for images in the lower panels. (J) Quantification of HK2 expression in TSPO-KO and WT gliomas shown in (I). (K) Measurement of glycolytic activities in TSPO-KO GL261 cells treated with the HIF-1α inhibitor PX-478 hydrochloride. (L) Quantification of glycolytic activities in (K). Three independent experiments were performed. The data are presented as means ± SEM. *P < 0.05, **P < 0.01, and n.s., not significant as determined using Student’s t-test (B, D, F, H, J, and L).