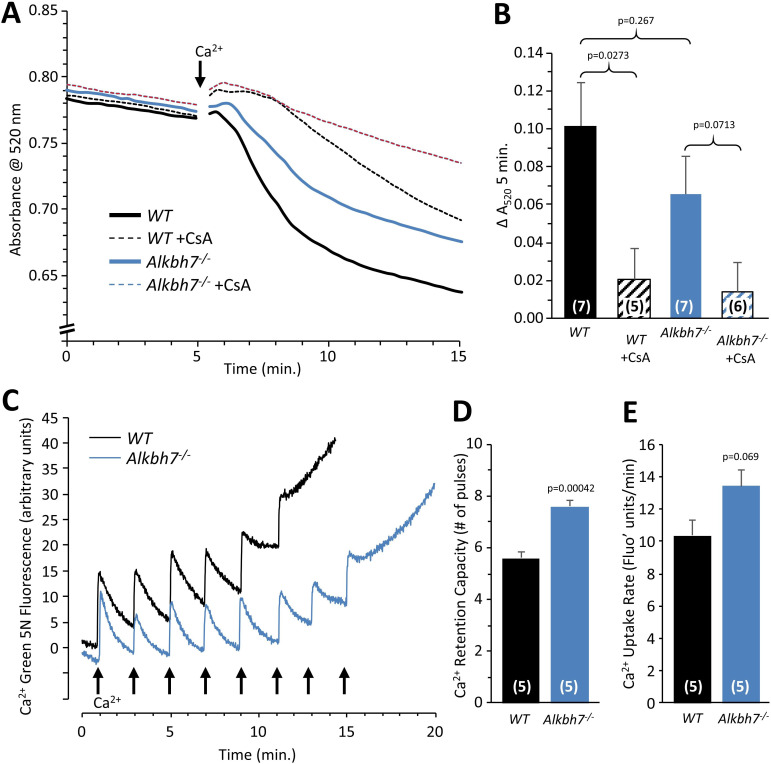
Figure 5. Mitochondrial PT pore and Ca2+ handling in WT vs.Alkbh7-/-.
(A) Opening of the mitochondrial PT pore was assayed spectrophotometrically in isolated cardiac mitochondria from young male WT and Alkbh7-/- mice. Average traces are shown, with addition of 100 µM Ca2+ to initiate PT pore opening and swelling indicated by the arrow. Dotted lines indicate the presence of the PT pore inhibitor cyclosporin A (CsA). Error bars are omitted for clarity. (B) Quantitation of pore opening, as the change in swelling (absorbance at 520 nm) in 5 min. Data are means ± SE, N = 7, with significance between groups (unpaired t-test) shown above error bars. (C) Mitochondrial Ca2+ handling assayed by Ca2+ green-5N fluorescence. Isolated cardiac mitochondria from young male WT and Alkbh7-/- mice were incubated with Ca2+ green-5N to indicate extra-mitochondrial [Ca2+]. Pulses of 10 µM Ca2+ were added at ~2 min intervals as indicated by arrows. Representative traces are shown. (D) Quantitation of the number of Ca2+ pulses tolerated by mitochondria before PT pore opening occurred (as indicated by a sharp upward deflection in the Ca2+ green-5N trace). (E) Quantitation of the initial rate of mitochondrial Ca2+ uptake, calculated from the downward slope in Ca2+ green-5N fluorescence on the first 3 Ca2+ pulses. Bar graphs in panels B/D/E show means ± SE, N = 5–7, with p-values (unpaired t-test) shown above error bars. In bar graphs, N for each group is shown in parentheses.