Figure 1: AXL targeting overcomes cetuximab resistance.
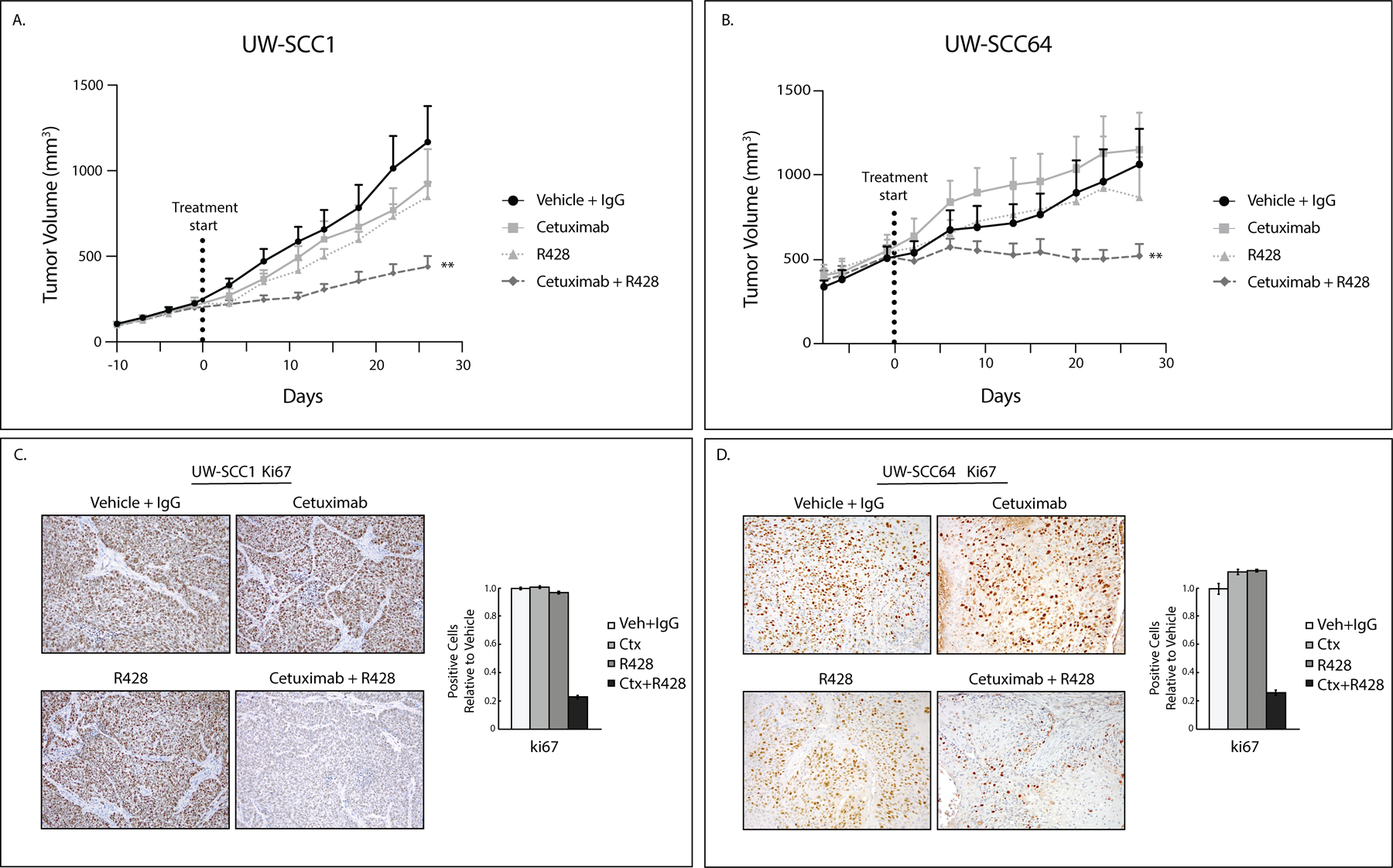
A, B: UWSCC PDXs (1 and 64) were implanted into flanks of nude mice and treated with vehicle (oral gavage, methylcellulose, twice daily) and human IgG (intraperitoneal, twice weekly) (UW-SCC1 n=9, UW-SCC64 n=13), cetuximab (intraperitoneal, 0.2 mg/mouse, twice weekly) (UW-SCC1 n=9, UW-SCC64 n=11), R428 (oral gavage, 25mg/kg, twice daily) (UW-SCC1 n=10, UW-SCC64 n=10), or combination of cetuximab with R428 for 28 days (UW-SCC1 n=9, UW-SCC64 n=14). Data was analyzed using linear mixed models where volume was modeled on natural-log scale. **, P<.01 for UW-SCC1 combination compared to single treatments and UW-SCC64 combination compared to cetuximab only.
C, D: Tumors from studies shown in 1A and 1B were stained for Ki67 using IHC. Image quantification shown.
