Figure 4: c-ABL targeting overcomes cetuximab resistance.
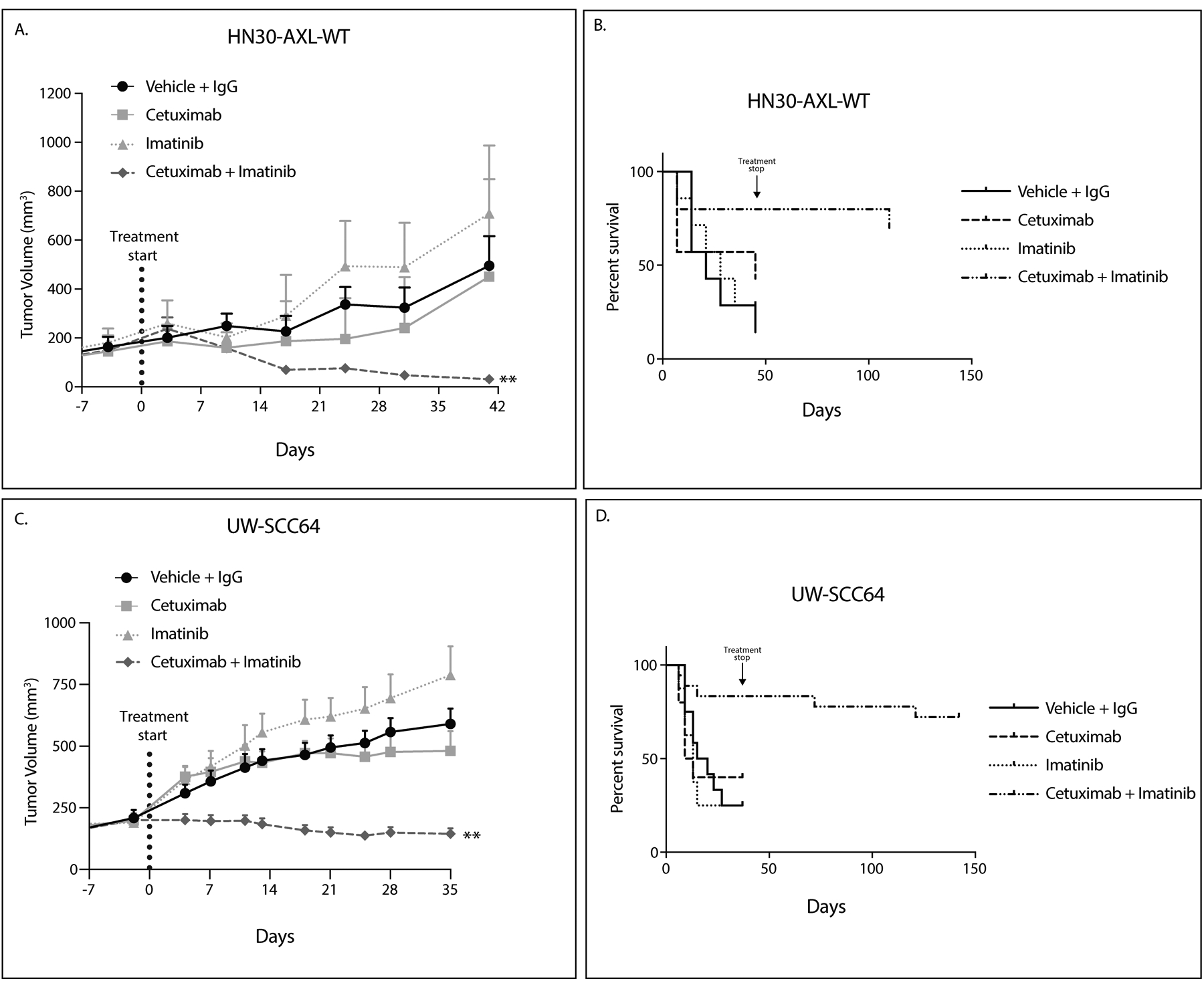
A,C: HN30-AXL-WT cells (A) or PDX UW-SCC64 (C) were implanted into flanks of nude mice and treated with vehicle (oral gavage, methylcellulose, twice daily) and human IgG (intraperitoneal, twice weekly) (HN30-AXL n=7, UW-SCC64 n=12), cetuximab (intraperitoneal, 0.2 mg/mouse, twice weekly) (HN30-AXL n=7, UW-SCC64 n=10), imatinib (oral gavage, 30mg/kg, once daily) (HN30-AXL n=7, UW-SCC64 n=8), or combination of cetuximab with imatinib (HN30-AXL n=10, UW-SCC64 n=18). Data was analyzed using linear mixed models. Volume data was untransformed given the presence of fully shrank tumors, resulting in volume readings of zero. **, P<.01 for combination compared to single treatments.
B, D: Data for studies in panels A and C are presented regarding overall survival. Survival was evaluated using tumor doubling time with “death” occurring when a tumor reached double its original volume. For HN30-AXL-WT, 5 of 10 tumors were still undetectable at 69 days after treatment was stopped. For UW-SCC64, 12 of 18 tumors were still not visible 107 days after treatment was stopped.
