Figure 2. Characterization of a small-molecule activator of LYPLAL1.
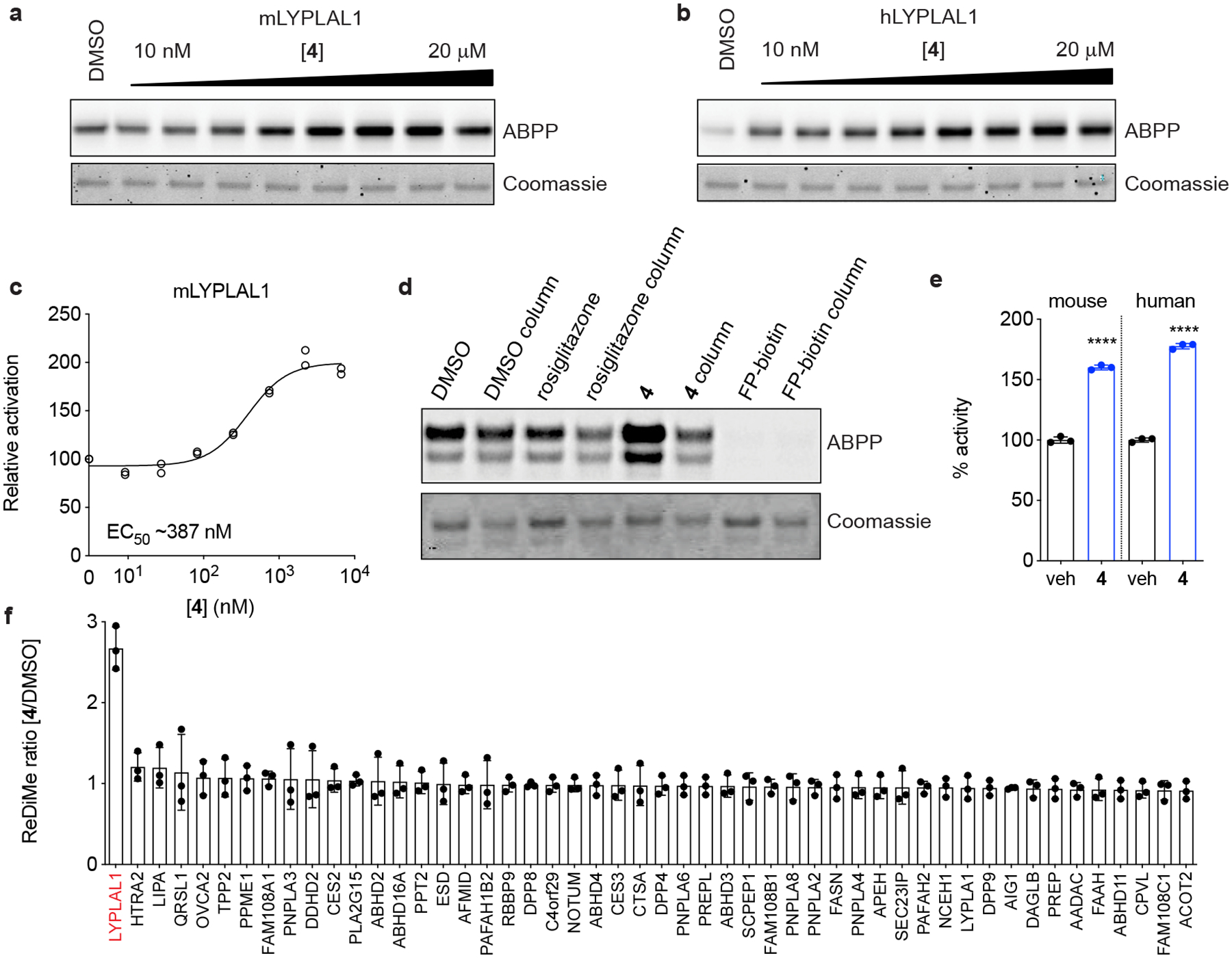
Purified (a) mouse or (b) human LYPLAL1 protein was incubated with increasing concentrations of 4 prior to labeling with FP-rhodamine and gel-based ABPP analysis. (c) Quantification of a dose response of 4 on mLYPLAL1 (n=2). (d) mLYPLAL1 was incubated with DMSO, 10 μM 4, 10 μM rosiglitazone (negative control) or 10 μM FP-biotin (covalent inhibitor). Reactions were split, and half the volume passed through a gel filtration column prior to labeling with FP-rhodamine and gel-based ABPP analysis. Loss of increased FP-rhodamine labeling in the gel filtration sample shows that 4 is a reversible activator. (e) Compound 4 (10 μM) increases the ability of mouse and human LYPLAL1 to hydrolyze the synthetic substrate PNPA (4-nitrophenyl acetate) (n=3; ****p<0.0001 two-tailed t-test). (f) Compound 4 selectively activates endogenous LYPLAL1. Total proteomes from HepG2 cells were treated with 10 μM 4 for 1 h and then labeled with FP-alkyne for 20 min prior to attachment of an azide-biotin tag and ABPP-MudPIT analysis (n=3). Error bars represent mean ± s.d. Representative results from two (d-f) or three (a-c) independent experiments; similar results were obtained in all experiments. In c, e, and f, n represents independent samples. Uncropped gels/blots for a, b, and d are shown in Supplementary Fig. 6.
