Figure 1.
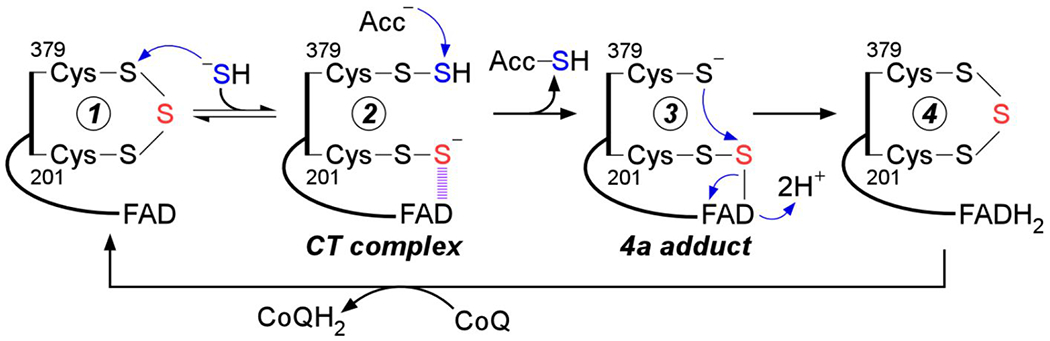
Postulated mechanism for sulfide oxidation catalyzed by human SQOR. Sulfide adds into the resting cysteine trisulfide (1) to generate a 379Cys-SSH persulfide and a 201Cys-SS− persulfide, with the latter participating in a CT complex with FAD (2). Sulfur transfer to a small molecule acceptor proceeds through a putative 4a adduct (3) to generate the reduced enzyme (4). Electron transfer from FADH2 to CoQ regenerates the resting enzyme. The oxidized sulfur and bridging sulfur in the cysteine trisulfide are labeled in blue and red, respectively.
