Figure 6.
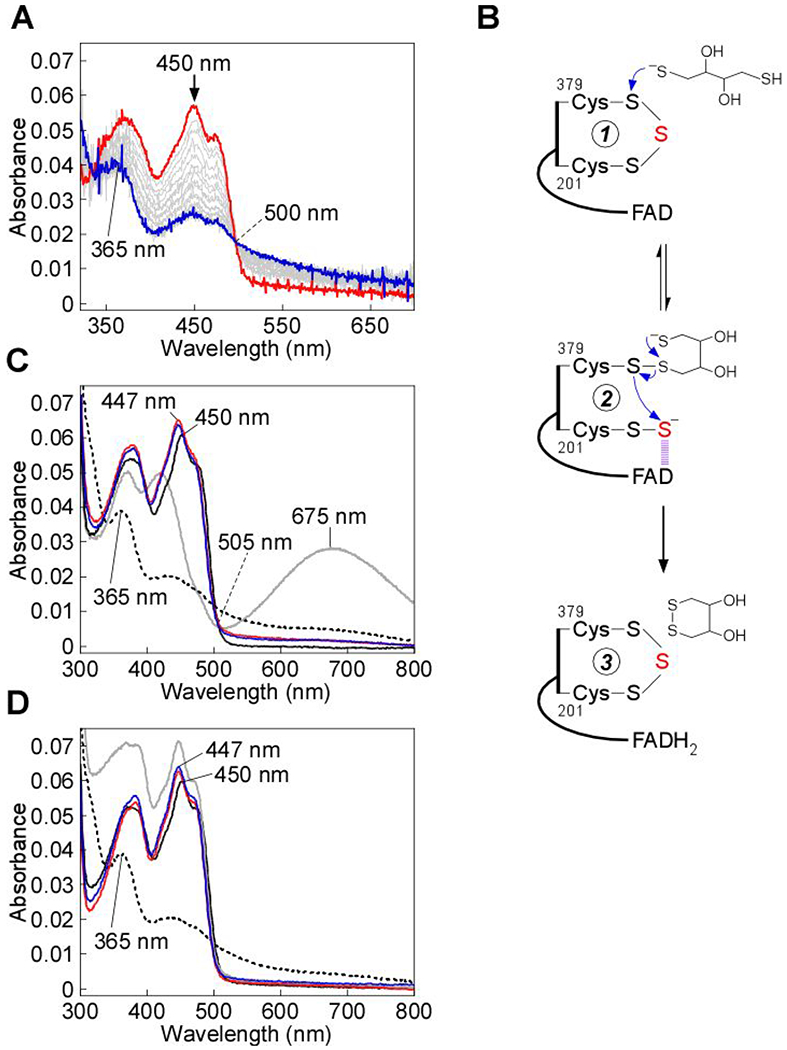
Dithiol-mediated reduction of FAD in SQOR. A, SQOR (10 μM) in Buffer A was rapidly mixed 1:1 (v/v) with DTT (400 μM) and FAD reduction was monitored over 7 s at 4 °C. B, Proposed mechanism for the addition of DTT into the SQOR cysteine trisulfide, leading to FAD reduction. DTT adds into the cysteine trisulfide (1) at the solvent-accessible Cys-379 to generate a mixed disulfide and 201Cys-SS− (2). An intramolecular thiol-disulfide exchange then regenerates the SQOR cysteine trisulfide, with electrons moving into FAD (3). C, SQOR (5 μM) in Buffer A (solid black line), was treated with DTT (200 μM), leading to FAD reduction (dashed black line), or β-mercaptoethanol (200 μM), leading to stable CT complex formation (solid gray line). FAD reduction was not observed in cyanide pre-treated SQOR (5 μM, solid red line) upon treatment with DTT (200 μM, solid blue line). D, SQOR (5 μM) under the same conditions as in (C) (solid black line), was treated with DHLA (200 μM), leading to FAD reduction (dashed black line), followed by re-oxidation by addition of CoQ1 (180 μM, solid gray line). FAD reduction was not observed in cyanide pre-treated SQOR (5 μM, solid red line) upon treatment with DHLA (200 μM, solid blue line). The data are representative of three independent experiments.
