Figure 9.
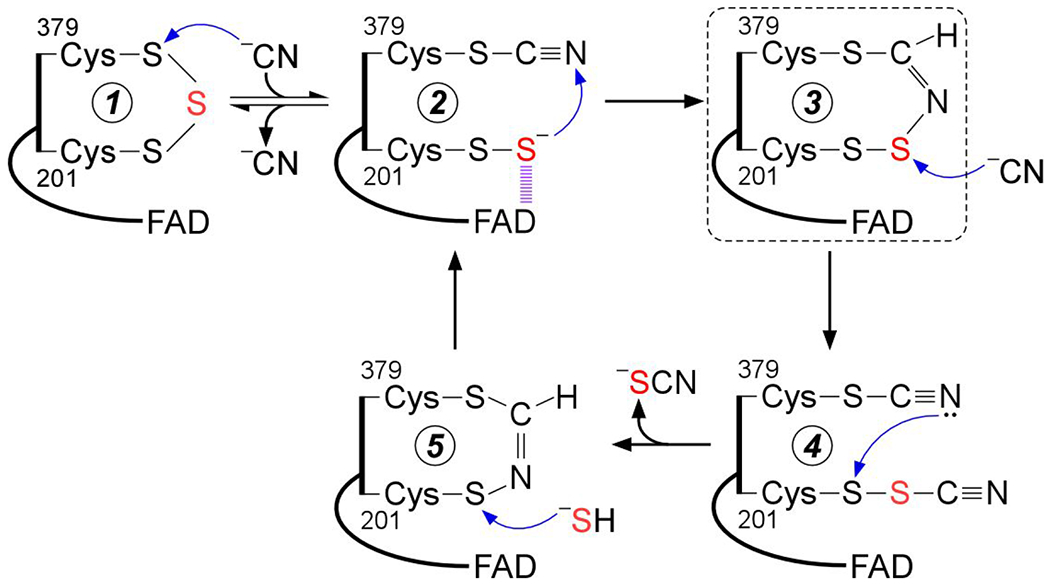
Proposed mechanism for cyanolysis and cysteine trisulfide rebuilding in SQOR. Cyanide adds into the resting cysteine trisulfide (1) to generate a 379Cys-S-CN organic thiocyanate while the bridging sulfur is retained in the 201Cys-SS− persulfide that participates in a CT complex with FAD (2). Conversion to the 379Cys N-(201Cys-disulfanyl)-methanimido thioate intennediate (3) leads to loss of the CT complex. Addition by a second cyanide at the sulfane sulfur of Cys-201 leads to intennediate (4), which can cyclize and eliminate thiocyanate (5), completing the cyanolysis reaction. Addition of sulfide to the Sγ of Cys-201 in the 379Cys N-(201Cys-sulfanyl)-methanimido thioate intennediate (5) regenerates the CT complex (2). Elimination of cyanide regenerates the resting trisulfide fonn of the enzyme. The bridging sulfur of the cysteine trisulfide is labeled in red. The dashed box highlights the intennediate observed in the crystal structure.
