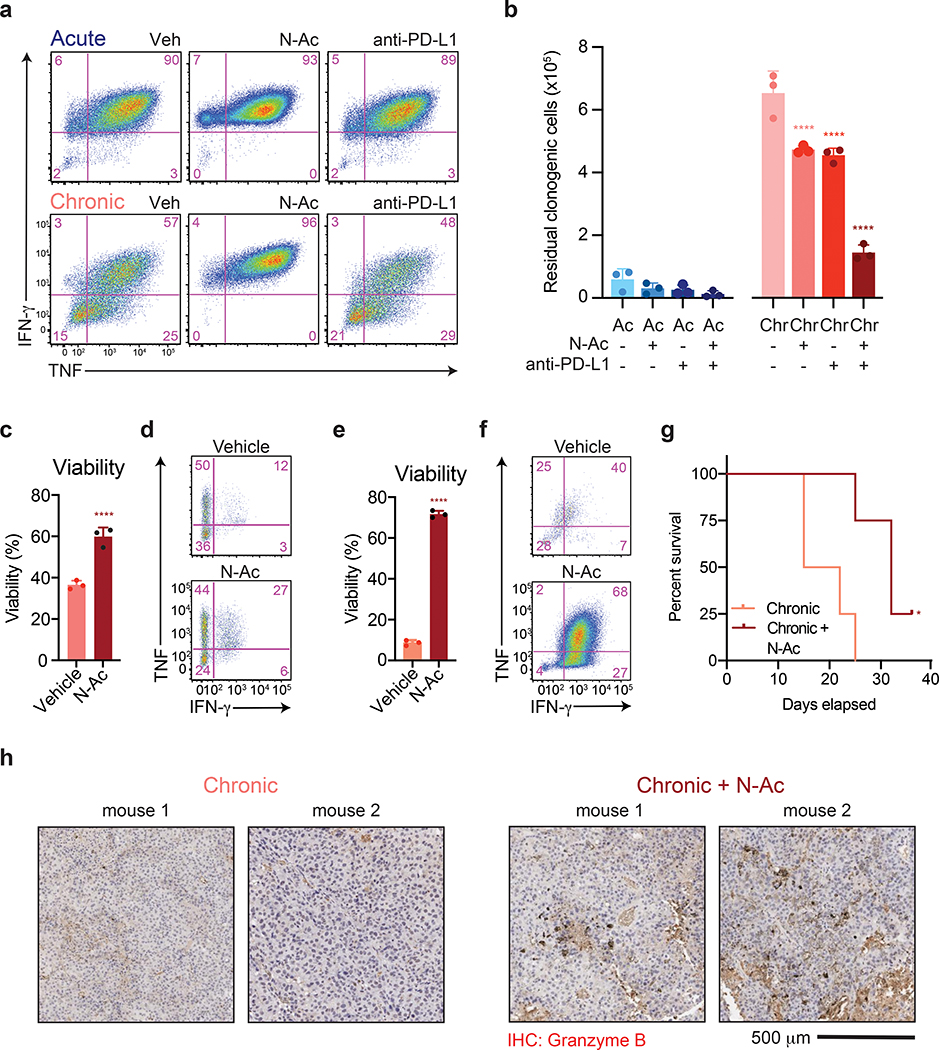
Figure 7. Antioxidants reverse chronic stimulation-driven loss of T cell effector function.
(a) Intracellular accumulation of IFN-γ and TNF following re-stimulation with PMA and ionomycin of T cells following acute or chronic stimulation in the presence or absence of N-AC or anti-PD-L1 as indicated. (b) Quantification of clonogenic B16 cells following 24 h of co-culture with acutely or chronically stimulated OT-I T cells that had been treated with N-AC or anti-PD-L1 throughout the co-culture period. (c-d) Viability (c) and intracellular production of IFN-γ and TNF (d) of CD8+ T cells isolated from EL4 tumors 3 days after re-stimulation in the presence or absence of N-AC. (e-f) Viability (e) and production of IFN-γ and TNF (f) by CAR-T cells 3 days after re-stimulation in the presence or absence of N-AC. (g) Kaplan-Meier curve showing survival of B16-ova-bearing recipient mice following adoptive transfer of OT-I T cells that had been chronically stimulated in the presence or absence of N-AC. All mice received anti-PD-L1 therapy twice weekly. (h) Immunohistochemistry showing enhanced Granzyme B expression in tumor-infiltrating T cells treated with N-AC. Staining of tumors extracted from two individual mice are shown. P values were calculated by one-way ANOVA with Sidak’s multiple comparisons post-test (b), unpaired, two-sided Student’s t-test (c,e) or log-rank (Mantel-Cox) test (g) relative to vehicle-treated cells. Data are presented as the mean ± s.d. of n=3 biologically independent samples or n=5 independent mice (g) from a representative experiment. *P<0.05, ****P<0.0001.