Abstract
Adolescent gastric cancers are extremely rare with a reported incidence of 0.05–0.10% in North America. We present a de novo case of gastric carcinoma in a 17-year-old teenager with no concomitant family history or risk factors. His main clinical presentation included anaemia and melaena stools. Despite an extensive clinical workup that included a diagnostic laparoscopy, the tumour was deemed surgically irresectable, and he was started on a palliative chemotherapy protocol at the local paediatric oncology centre. He demised 7 months later. This is the first recorded case of an adolescent gastric cancer in Saskatchewan, Canada. This case highlights the need for an international tumour registry to document and investigate rare adolescent gastric malignancies and thereby potentiate a possible cure through the pooling of limited resources.
Keywords: adolescent gastric cancer, paediatric malignancy, paediatric tumour registry
INTRODUCTION
Gastric adenocarcinoma is an extremely rare subtype of adolescent malignancy, with the current literature describing an incidence in North America of 0.05–0.10% of all adolescent neoplasms [1–3]. Signet ring adenocarcinoma comprises 45% of adolescent gastric adenocarcinoma cases [3]. This documented rarity of gastric adenocarcinoma in the adolescent population results in a delayed diagnosis, with subsequent investigations invariably revealing an advanced pathological staging [3]. We describe a case of a signet ring adenocarcinoma in a 17-year-old male, the first documented case, to our knowledge, in Saskatchewan, Canada [4] (Tables 1 and 2).
Table 1.
The incidence of stomach cancer in Canadians aged 10–14 from 2010 to 2017 [4]. No data available for 2018–2020
| Incidence of stomach cancer in Canadians aged 10–14 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
| Newfoundland and Labrador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince Edward Island | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Brunswick | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quebec | 0 | .. | .. | .. | .. | .. | .. | .. |
| Ontario | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Manitoba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Saskatchewan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alberta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| British Columbia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northwest Territories | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nunavut | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table 2.
The incidence of stomach cancer in Canadians aged 15–19 from 2010 to 2017 [4]. No data available for 2018–2020
| Incidence of stomach cancer in Canadians aged 15–19 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
| Newfoundland and Labrador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince Edward Island | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Brunswick | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quebec | 0 | .. | .. | .. | .. | .. | .. | .. |
| Ontario | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 0 |
| Manitoba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Saskatchewan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alberta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| British Columbia | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northwest Territories | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nunavut | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
CASE REPORT
A 17-year-old Indigenous male presented to our emergency department with a complaint of melaena stools for the past 3 months. He denied a history of haematemesis, haematochezia and previous surgeries and had no known allergies. His past medical history comprised of one previous emergency room visit a month earlier at symptom onset which required a blood transfusion for a haemoglobin level of 7.0 mg/dl. Blood parameters at his initial presentation demonstrated iron deficiency anaemia, although the aetiology for the anaemia was never ascertained. His family history was unremarkable with no history of gastric cancers and gastrointestinal malignancies or personal history of malignancy.
Clinically, the patient was emaciated, with a scaphoid abdomen, and extremely pale. He did not appear jaundiced, and a digital rectal exam yielded fresh melaena stools. Physical examination disclosed no evidence of palpable lymph nodes or abdominal masses. His haemoglobin level was 7.5 mg/dl and he was transfused two units of packed cells. The complete blood count showed an iron deficiency anaemia once again, and his expanded biochemistry profile was normal.
Urgent gastroscopy revealed a giant gastric ulcer in the antrum (Fig. 1) which was biopsied and sent for pathological investigation. The ulcer displayed no bleeding stigmata. He was started on eradication therapy for Helicobacter pylori. Pathology revealed a signet ring adenocarcinoma of the stomach (Fig. 2A and B). A computerized tomography (CT) scan (Fig. 3) showed a large mass in the distal stomach, and the ensuing positron emission tomography (PET) scan (Fig. 4) showed enlarged D1 perigastric lymph nodes with no obvious metastatic disease.
Figure 1.

A giant gastric ulcer in the antrum during gastroscopy (orange arrow).
Figure 2.

(A) Mucicarmine stain with diffuse sheets of malignant cells and rare glandular formation. The mucin is intracytoplasmic. (B) Pankeratin immunohistochemistry with positive Pankeratin confirms the epithelial linage of the malignant infiltration.
Figure 3.
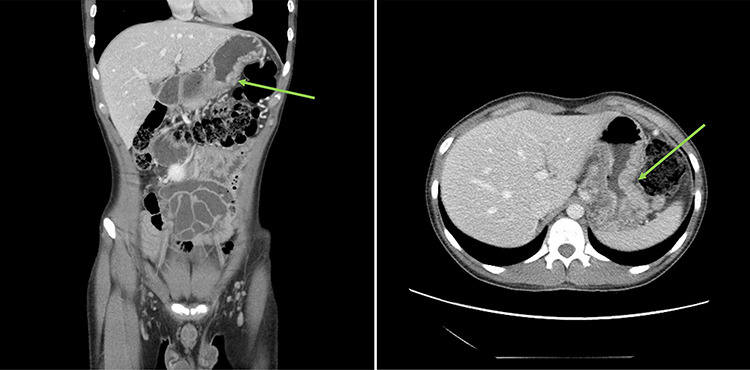
: (A and B) CT scans (coronal and axial views) showing a large mass in the distal stomach (green arrows).
Figure 4.

(A and B) PET scans (axial and coronal views) showing enlarged D1 perigastric lymph nodes (green arrows) and no apparent metastatic disease.
At diagnostic laparoscopy, the tumour was deemed surgically irresectable with large clinically visible perigastric lymph nodes (Fig. 5). The patient was referred to surgical oncology for neoadjuvant chemotherapy on a palliative protocol. The patient succumbed 7 months later in the hospital.
Figure 5.

Clinically irresectable gastric tumour with large, visible perigastric lymph nodes, visualized on diagnostic laparoscopy (green arrow).
DISCUSSION
Adolescent gastric cancers are a rare affliction, with only 25 cases recorded in Canada since 2010 [4] (Tables 1 and 2). No previous cases have been described in Saskatchewan, although the provinces of Ontario, British Columbia and Manitoba have all recorded cases during this period [4]. In the adult demographic, risk factors such as H. pylori infection, atrophic gastritis, smoking, male sex, advanced age and positive family history are associated with a higher risk of gastric adenocarcinoma development [5]. Traditional risk factors are unreliable diagnostic indicators in the adolescent population as gastric adenocarcinomas manifest as de novo occurrences expressed via genetic mutations or develop as a side effect of gastric lymphoma treatment [1, 6, 7]. Recent literature suggests that adolescents with de novo gastric carcinomas behave oncologically different than their adult counterparts. This is demonstrated by a 2-fold increase in SRA risk, a significantly lower proportion of primary tumours in the gastric cardia, advanced staging at diagnosis, frequent nodal involvement and poorly differentiated histology [3, 8].
The presenting symptoms of adolescent gastric cancers are typically vague or flu-like on initial presentation. Symptoms include abdominal pain, anorexia, haematemesis, melaena, haematochezia, variable mass effect symptoms and a palpable abdominal mass [2, 6, 9]. Treatment of adolescent gastric adenocarcinoma includes prompt surgical resection of operable tumours with concurrent chemotherapy and radiation [3]. Adolescents typically present with aggressive, advanced tumours but are usually healthy and able to withstand more invasive interventions than their adult counterparts with indolent disease, resulting in a comparable survival rate between the two population groups. Regardless of age, gastric adenocarcinoma is associated with a poor prognosis, and the median survival in North America is 5 months from the initial diagnosis [10].
The poor prognosis associated with a diagnosis of gastric adenocarcinoma necessitates early gastroscopy and biopsy in adolescent patients presenting with concerning gastrointestinal symptoms to expedite diagnosis and treatment. This must be accompanied by a sharp clinical acumen, as these symptoms may initially appear benign. The sinister onset of symptoms and poor prognosis in adolescents suggest that clinical signs may be an augury of a rapidly growing, irresectable, advanced stage entity. This was certainly the case with our patient.
To prevent such senseless loss in the future, clinicians require considerably more than apt clinical judgement and a functional CT scanner. An international tumour registry for rare paediatric and adolescent cancers is an essential first step in the race to overcome these unusual and aggressive malignancies.
Resected specimens should be sent for genetic screening and cataloguing which can help ascertain risk factors that predispose certain adolescents to developing uncommon cancers [1, 10]. Identifying these adolescent, at-risk patients early is important to provide them and their families with all possible treatment options from the onset of disease. This would help reduce the disease burden on this population cohort and possibly contribute to a potential cure in the near future (1, 10). This pooling of medical resources will stand us in good stead given the extremely low incidence of adolescent malignancies which precludes the ability for one group of physicians to study these tumours closely.
CONCLUSION
We present the first recorded case of an adolescent gastric cancer in Saskatchewan, Canada, and strongly advocate for the formation of an adolescent tumour registry.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
Contributor Information
Lara Witt, College of Medicine, University of Saskatchewan, Saskatoon, SK, Canada.
Yagan Pillay, Department of Surgery, University of Saskatchewan, Victoria Hospital, Prince Albert, SK, Canada.
Rathi M Sabaratnam, Department of Pathology and Laboratory Medicine, Victoria Hospital, Prince Albert, SK, Canada.
Richard J Bigsby, Department of Thoracic Surgery, University of Saskatchewan, Saskatoon, SK, Canada.
References
- 1. Subbiah V, Varadhachary G, Herzog CE, Huh WW. Gastric adenocarcinoma in children and adolescents. Pediatr Blood Cancer 2011;57:524–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGill TW, Downey EC, Westbrook J, Wade D, Garza J.. Gastric carcinoma in children. J Pediatr Surg 1993;28:1620–1. [DOI] [PubMed] [Google Scholar]
- 3. Tessler RA, Dellinger M, Richards MK, Goldin AB, Beierle EA, Doski JJ, et al. Pediatric gastric adenocarcinoma: a National Cancer Data Base review. J Pediatr Surg 2019;54:1029–34. [DOI] [PubMed] [Google Scholar]
- 4. Statistics Canada Number and rates of new cases of primary cancer, by cancer type, age, group and sex. 17 February 2020.
- 5. Patiroglu T, Eke Gungor H, Arslan D, Deniz K, Unal E, Coskun A. Gastric signet ring carcinoma in a patient with ataxia-telangiectasia: a case report and review of the literature. J Pediatr Hematol Oncol 2013;35:e341–3. [DOI] [PubMed] [Google Scholar]
- 6. Harting MT, Blakely ML, Herzog CE, Lally KP, Ajani JA, Andrassy RJ. Treatment issues in pediatric gastric adenocarcinoma. J Pediatr Surg 2004;39:e8–10. [DOI] [PubMed] [Google Scholar]
- 7. Worthley DL, Phillips KD, Wayte N, Schrader KA, Healey S, Kaurah P, et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut 2012;61:774–9. [DOI] [PubMed] [Google Scholar]
- 8. Pisanu A, Podda M, Cois A, Uccheddu A. Gastric cancer in the young: is it a different clinical entity? A retrospective cohort study. Gastroenterol Res Pract 2014;2014:125038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Periasamy K, Radhakrishna N, Mukherji A, Ganesh RN. Gastric adenocarcinoma in a 16-year-old female. Ochsner J 2018;18:395–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pashankar F, Bisogno G, Ribeiro R, Messinger Y, Schultz K, Rodriguez-Galindo C. The role of registries and tumor banking in rare pediatric tumors. Current Pediatrics Reports 2015;3:128–36. [Google Scholar]


