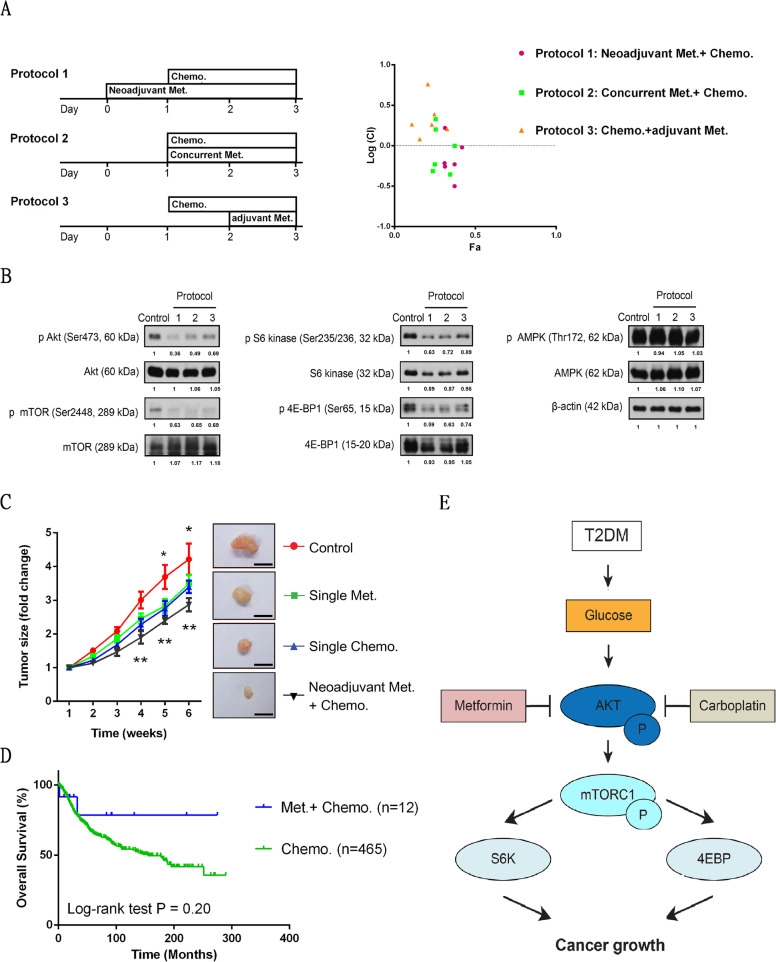
Fig. 4.
The Beneficial Synergistic Effects of Neoadjuvant Metformin under Combination Treatment. a and b Protocol 1: a neoadjuvant protocol, MOSECs treated with metformin alone for 1 day and then with a combination of metformin and carboplatin for 2 days. Protocol 2: a concurrent protocol, MOSECs treated with both metformin and carboplatin from day 2 for 2 days. Protocol 3: an adjuvant protocol, MOSECs treated with carboplatin alone from day 1 for 1 day and then with a combination of metformin and carboplatin for 1 day. Pink circles represent log (CI) values under protocol 1, green squares represent those under protocol 2, and yellow triangles represent those under protocol 3 (concentration of metformin: 0.25 and 0.5 mM; concentration of carboplatin: 5, 10 and 50 μM). Met.: metformin; Chemo.: chemotherapy. ImageJ analysis for relative intensity of protein bands. c MOSECs were injected into B6 mice (n = 20), which were divided into 4 groups, and subcutaneous tumor size was measured after different treatments (control, metformin or carboplatin alone, neoadjuvant metformin from Monday combined with carboplatin from Wednesday). Subcutaneous tumors were assessed at the end of the experiment. *: Control vs. Met.; p < 0.05. ** Control vs. Chemo., and Met. + Chemo.; p < 0.01. d Kaplan-Meier OS of ovarian cancer patients with (n = 12) or without (n = 465) metformin, in (before or during) chemotherapy. e The model of synergistic inhibitory effects by neoadjuvant metformin combined with chemotherapy in the AKT/mTOR pathway