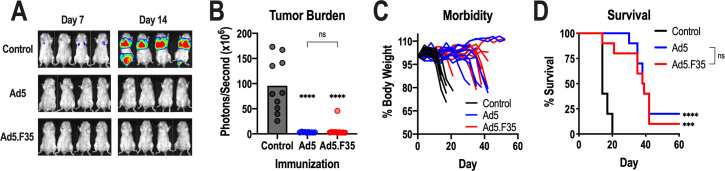
Figure 3.
Antitumor efficacy of Ad5-GUCY2C-S1 and Ad5.F35-GUCY2C-S1. (A–D) BALB/c mice (n=10 mice/group) were immunized intramuscularly with control or 1010 vp of Ad5-GUCY2C-S1 or Ad5.F35-GUCY2C-S1 and challenged 7 days later with a mouse colorectal cancer cell line, CT26, expressing GUCY2C and luciferase. On days 7 and 14 following challenge, mice were injected with D-luciferin and imaged (A) to quantify tumor burden (day 14; B). Mice were weighed twice weekly (C) and monitored for survival (D). Tumor burden (B) was analyzed by one-way analysis of variance and survival comparisons (D) were analyzed by the Mantel-Cox log-rank test. In (B) and (D), asterisks (*) indicate comparisons of GUCY2C vaccines to the control and brackets (]) indicate comparisons between Ad5 and Ad5.F35 vaccines. ns, not significant; Ad5, adenovirus serotype 5.