Abstract
Introduction
Host immunity plays a vital role in tumorigenesis, including in tumor invasion and metastasis. However, the precise underlying mechanism remains to be explored. The enzyme 15-PGDH, which plays a key role in prostaglandin degradation, is a critical inflammatory mediator in gastric cancer (GC) tumorigenesis.
Materials and Methods
Immunohistochemistry was performed to determine 15-PGDH expression in GC and the corresponding adjacent non-neoplastic tissues (n=92).
Results
The expression of 15-PGDH in GC tissues was significantly lower than that in paracancerous tissues (P<0.001) and found to correspond inversely with GC differentiation (P=0.043) and lymph node metastasis (P=0.046). In contrast, FOXP3 expression was increased in poorly differentiated GC tissues (P=0.001). Kaplan–Meier analysis revealed that GC patients with low expression of 15-PGDH (Log rank test, P=0.007) and high expression of FOXP3 (Log rank test, P=0.009) had shorter overall survival (OS) than those with high 15-PGDH and low FOXP3 expression. OS was also correlated with pathological tumor-node-metastasis stage (Log rank test, P=0.014). Furthermore, using Cox proportional hazard regression, 15-PGDH expression [hazard ratio (HR): 0.605 (0.440–0.833); P=0.002] was identified as an independent factor for OS.
Conclusion
Our data suggest that 15-PGDH may contribute to anti-tumor immunity by regulating FOXP3+ Treg cells. The findings are useful for the identification of therapeutic targets for the management of GC.
Keywords: gastric cancer, 15-PGDH, immunosuppression, FOXP3, Tregs
Introduction
Gastric cancer (GC) is the fifth most common malignancy and the second leading cause of cancer-related death worldwide, partly because most patients are diagnosed at an advanced stage and only receive palliative care.1 Surgery, which is still the primary approach for GC patients in the early stages of the disease, offers a high rate of success. However, approximately 75% of GC patients are diagnosed at advanced stage, and the 5-year-survival rate among patients with local or lymph node metastasis is only 20%.2 Therefore, the identification of novel prognostic biomarkers and therapeutic targets in GC is critical.
The cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) pathway plays a key role in GC tumorigenesis, including in the promotion of proliferation and angiogenesis, inhibition of apoptosis, immunosuppression, invasion, and metastasis.3,4 Furthermore, 15-hydroprostaglandin dehydrogenase (15-PGDH), a natural antagonist against COX-2 function,5 is a key enzyme in PG degradation. The role of 15-PGDH in GC is controversial: some studies suggest that 15-PGDH acts as a tumor suppressor in GC,6,7 while others report that 15-PGDH is not altered in gastric carcinoma or associated with clinicopathologic parameters or prognosis.8,9 Hence, the correlation of 15-PGDH with gastric tumorigenesis remains to be clarified.
Host immunity plays a vital role in tumorigenesis in many cancers, during which the immune escape of tumor cells from tumor-infiltrating lymphocytes (TILs) represents a critical step in cancer invasion and metastasis.10 Previous studies suggest that the prognostic implications of TILs in GC depend on their density and type.11 CD4+/CD25+ regulatory T (Treg) cells suppress tumor progression, and play a major role in immune escape and suppression of the anti-tumor immune response, thereby promoting tumor growth and invasion.12,13 Tumor immunosuppression mediated by Treg cells in cancer patients is emerging as a topic of increasing interest in tumor research.14 Studies have demonstrated that Treg cells accumulate in peritumorous tissues in lung, gastric, intestinal, and liver cancers at higher levels than in healthy tissues, and that this is indicative of poor prognosis.15 However the precise mechanisms underlying the regulation of Treg cells in GC remain to be explored.
Forkhead Box Protein 3 (Foxp3), a transcription factor that regulates the development of Tregs, is considered to be a marker of these cells. It is proposed that tumor growth is due to the balance of inflammatory networks between that tumorigenesis non-specifically and specific anti-tumor activity.16 Therefore, given both their prognostic value and therapeutic potential, the identification of cytokines whose expression influences patient survival in GC is critical. PGE2 receptors are expressed on the surface of Treg cells; binding with COX-2 induces PGE2 to increase FOXP3 expression and enables T cells to acquire inhibitory activity. The COX-2/PGE2 signaling pathway enhances FOXP3 expression in Treg cells, particularly in GC tissues, and is associated with poor prognosis.17 However, the relationship between the expression level of FOXP3 and 15-PGDH in GC remains to be investigated.
In the current study, GC tissues were collected from patients along with extensive clinical data, including information for sex, age, tumor differentiation, tumor invasion depth, lymph node metastasis, and pTNM stage. Our data support the view that 15-PGDH plays a role in the suppression of the development of gastrointestinal cancers. The present findings may be useful for improving diagnosis, as well as defining a potential therapeutic target for precision medicine, in GC.
Materials and Methods
Patients
This was a retrospective study of a series of 92 GC patients who underwent gastrectomy between 2014 and 2018 at the Gansu Wuwei Tumour Hospital, Gansu, China. All cases were diagnosed with tubular adenocarcinoma, at advanced stage. None of the patients received chemotherapy or other forms of therapy before surgery. Gastric cancerous and corresponding adjacent (≥5 cm) non-tumor tissues were obtained, and data for the clinicopathological features of each patient were collected from the Department of Oncology, Gansu Wuwei Tumour Hospital (Table 1). The pathological tumor-node-metastasis (pTNM) staging of GC was performed according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM system for differentiated gastric carcinoma.18 The current study was approved by the Medical Ethics Committee of Gansu Wuwei Tumour Hospital, and written informed consent for the use of tissues for ex vivo experimentation was obtained from each patient prior to surgery. Follow-up duration was defined as the time from the diagnosis of GC until the final visit. Overall survival (OS) was defined as the time interval from the date of surgery to GC-related death, or the final visit.
Table 1.
Relationship of 15-PGDH Expression with Clinicopathologic Parameters and FOXP3 in GC
| n | 15-PGDH Expression | P-value | ||||
|---|---|---|---|---|---|---|
| 0 (n=6) | 1 (n=28) | 2 (n=15) | 3 (n=43) | |||
| Sex | ||||||
| Male | 71 | 4 | 23 | 12 | 32 | 0.796 |
| Female | 21 | 2 | 5 | 3 | 11 | |
| Age | ||||||
| <55 | 44 | 4 | 15 | 5 | 20 | 0.472 |
| ≥55 | 48 | 2 | 13 | 10 | 23 | |
| Differentiation | ||||||
| Tubular | 27 | 1 | 13 | 1 | 12 | 0.043 |
| Poor | 65 | 5 | 15 | 14 | 31 | |
| Invasion depth | ||||||
| T1/T2 | 21 | 0 | 5 | 3 | 13 | 0.311 |
| T3/T4 | 71 | 6 | 23 | 12 | 30 | |
| Lymph node metastasis | ||||||
| No | 55 | 2 | 12 | 11 | 30 | 0.046 |
| Yes | 37 | 4 | 16 | 4 | 13 | |
| TNM stage | ||||||
| I+II | 32 | 2 | 5 | 5 | 20 | 0.104 |
| III+IV | 60 | 4 | 23 | 10 | 23 | |
| FOXP3 expression | ||||||
| Low | 55 | 1 | 13 | 6 | 35 | 0.001 |
| High | 37 | 5 | 15 | 9 | 8 | |
Abbreviations: T2, tumour invasion of the muscularis propria or subserosa; T3, tumour invasion extends to or beyond the serosa; T4, tumour invasion of adjacent structures; Tubular, tubular adenocarcinoma; Poor, poorly differentiated adenocarcinoma.
Immunohistochemistry
A conventional immunohistochemical (IHC) staining protocol was used in this study. Briefly, paraffin-embedded tumor tissues were cut into 4-μm-thick sections, dried, deparaffinized, and dehydrated in a graded series of ethanol. Tissue sections were treated with 1% hydrogen peroxide for 10 min to block endogenous tissue peroxidase activity, followed by treatment with bovine serum for 30 min to reduce nonspecific binding. Antigen retrieval was then performed using citrate buffer (pH 6.0) using the following protocol: high-heat microwave processing for 5 min, followed by low-heat microwave processing for 20 min. All the slides were incubated with rabbit polyclonal anti-15-PGDH antibody (OM237529, 1:500 dilution, Omnim Abs, USA), and rabbit anti-human FOXP3 polyclonal antibody (OM279148, 1:100 dilution, marker for Tregs; Omnim Abs, USA) overnight at 4°C, followed by incubation for 30 min in Ultra-Sensitive S-P Kit (Maixin-Bio, Fuzhou, China). Slides were rinsed with phosphate-buffered saline before color development using a 3, 3ʹ-diaminobenzidine substrate kit, and then counterstained with hematoxylin.
Evaluation of Immunohistochemical Data
In order to assess the immunoreactivity of 15-PGDH and FOXP3, specimens were examined under an Olympus BX53 microscope by two senior pathologists, who were blinded to the clinicopathologic data; analysis was performed in a semi-quantitative manner, in terms of staining intensity and percentage of positive cells. Ten areas were randomly selected and counted at a magnification of ×200. In case of discrepancy, consensus was achieved using a multi-headed microscope. Specimens with cytoplasmic staining of 15-PGDH and FOXP3 antibodies in lymphocytes were defined as positive. IHC staining of 15-PGDH and FOXP3 protein was assessed in terms of staining intensity and percentage of positive cells as follows: 0 (negative), 1 (weak staining, ≤10% of cells staining positive), 2 (moderate staining, 10–90% of cells staining positive), and 3 (strong staining, >90% of cells staining positive). The final score for each slide was represented by the average of three representative high-power fields (hpf, ×400). Scores ≤1 was defined as indicating low expression, while those ≥2 were defined as indicative of high expression.
Statistical Analysis
Statistical analysis was performed using SPSS 21.0 software. The differences in 15-PGDH expression between gastric cancerous and paracancerous tissues were analyzed by Wilcoxon signed-rank tests. Correlation of 15-PGDH expression with clinicopathological features and FOXP3 expression in GC patients was analyzed by χ2 and Mann–Whitney U-tests. Furthermore, the correlation of OS with clinicopathological features and expression of 15-PGDH and FOXP3 was analyzed using the Kaplan–Meier method with the Log rank test. The Cox proportional hazard regression model was used to identify the prognostic factors that influenced OS.
Results
Demographic Information
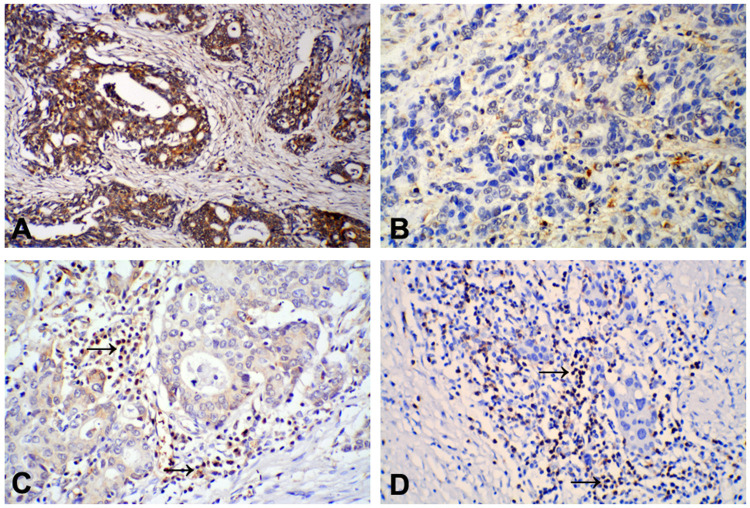
The demographic and clinicopathologic characteristics for the 92 primary GC patients and 92 matched non-cancer tissues are listed in Table 1; 71 of the patients were males and 21 were females, with a median age of 57 years (range: 32–78 years) at surgery. The median OS duration of the patients was 23 months (range: 1–53 months). Expression of 15-PGDH in 92 GC tissue samples and the 92 corresponding paracancerous non-tumor tissue samples were analyzed by IHC. Positive staining of the 15-PGDH protein was mainly observed in the cytoplasm of both tumor and normal gastric cells, and 15-PGDH expression in high/moderately differentiated GC was found to be higher than in poorly differentiated GC (Figure 1A and B). Based on 15-PGDH immunoreactivity, 34 (37.0%) of GC tissue samples exhibited low 15-PGDH expression and 58 (63.0%) exhibited high 15-PGDH expression; in comparison, among the paracancerous tissues, 21 (22.8%) exhibited low 15-PGDH expression and 71 (77.2%) exhibited high 15-PGDH expression. Positive staining of FOXP3 protein was mainly observed in the nuclei of lymphocytes in intratumoral tissues: FOXP3+ Tregs were less abundant in high/moderately differentiated GC than in poorly differentiated GC tissues (Figure 1C and D).
Figure 1.
Positive staining of 15-PGDH protein was mainly found in the cytoplasm of gastric cancer (GC) tumor cells: (A) High/moderately differentiated GC, positive staining; (B) Poorly differentiated GC, negative staining. IHC staining of FOXP3 expression was mainly observed in the cytoplasm of lymphocytes in gastric cancer tissues: FOXP3 expression in high/moderately differentiated GC was lower (C) than in poorly differentiated GC (D) tissues. (×200).
Correlation of 15-PGDH Expression with Clinicopathological Features and FOXP3 Expression in GC
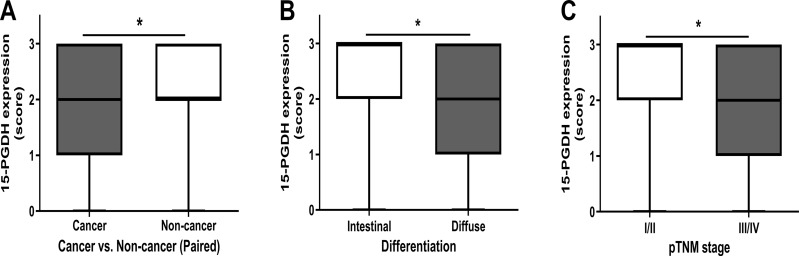
The expression of 15-PGDH in GC tissues was significantly lower than in paracancerous non-tumor tissues (P<0.001) (Table 2). Quantitative analysis showed that 15-PGDH expression was lower in GC tissues than in the corresponding non-tumor tissues (Figure 2A; P<0.05). The correlation of 15-PGDH expression with clinicopathological features was analyzed. Our data showed that 15-PGDH expression was higher in the intestinal GC than in the poorly differentiated GC (Figure 2B; P<0.05). Furthermore, 15-PGDH expression was significantly lower in III/IV GC than in I/II GC (Figure 2C; P<0.05). GC patients with tubular differentiation (P=0.043) and lymph node metastasis (P=0.046) exhibited lower 15-PGDH expression; however, 15-PGDH expression was not associated with sex, age, invasion depth, or pTNM stage (all P>0.05). Furthermore, the expression of FOXP3 was inversely correlated with that of 15-PGDH (P=0.001) (Table 1).
Table 2.
Relationship of 15-PGDH Expression Between Non-Cancer and Gastric Cancer
| 15-PGDH Expression | P-value | ||||
|---|---|---|---|---|---|
| 0, n (%) | 1, n (%) | 2, n (%) | 3, n (%) | ||
| Non-cancer (n=92) | 14 (15.2) | 7 (7.6) | 43 (46.7) | 28 (30.5) | <0.001 |
| Gastric cancer (n=92) | 6 (6.5) | 28 (30.5) | 15 (16.3) | 43 (46.7) | |
Figure 3.
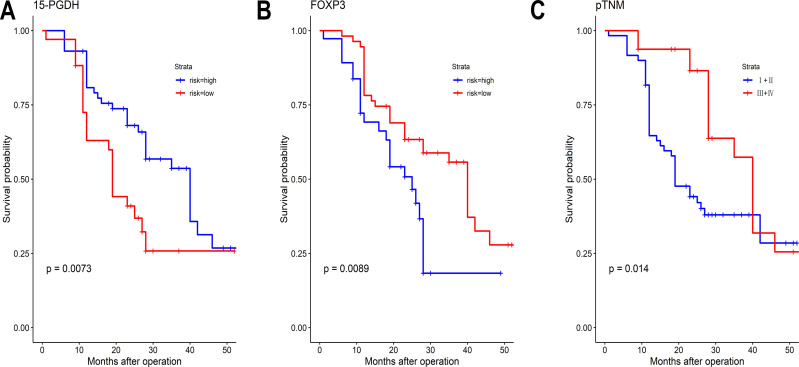
Kaplan-Meier survival curves for patients according to: (A) High or low 15-PGDH expression (B) High or low FOXP3 expression, and (C) pTNM stage.
Figure 2.
Comparison of 15-PGDH expression between paired cancer vs non-tumor tissue samples in each patient (A) by using Wilcoxon signed-rank test. Comparison of 15-PGDH expression between various differentiation (B) and pTNM stages (C) by using Mann–Whitney U-test. *P<0.05.
Univariate and Multivariate Analyses of the Relationship Between GC Patient Survival and 15-PGDH Expression
Univariate analysis was performed to determine whether various factors (15-PGDH, FOXP3, sex, age, differentiation, invasion depth, pTNM, and lymph node metastasis) influence survival rate (Table 3). Kaplan-Meier analysis revealed that patients with low expression of 15-PGDH (Log rank test, P=0.007), high expression of FOXP3 (Log rank test, P=0.009), and higher pTNM stage (Log rank test, P=0.014) had a shorter OS than patients with high expression of 15-PGDH, low expression of FOXP3, and lower pTNM stage. The survival curves are shown in Figure 2. Multivariate analysis and the Cox proportional hazard regression method were further used to evaluate the risk factors that affect OS of GC patients. Among these variables, 15-PGDH [hazard ratio (HR): 0.605 (0.440–0.833); P=0.002] expression was identified as an independent predictive factor for OS (Table 4).
Table 3.
Predictive Factors for Overall Survival by Univariate Analysis in GC
| n | n of Event | Median OS (Range) | Log Rank Test, χ2 | P-value | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 71 | 41 | 28.0 (22.8–33.2) | 0.215 | 0.643 |
| Female | 21 | 11 | 40.0 (27.2–52.8) | ||
| Age | |||||
| <55 | 44 | 27 | 25.0 (18.3–31.7) | 0.808 | 0.369 |
| ≥55 | 48 | 25 | 28.0 (26.1–30.0) | ||
| Differentiation | |||||
| Tubular | 27 | 15 | 40.0 (31.4–48.7) | 0.020 | 0.886 |
| Poor | 65 | 37 | 28.0 (27.0–29.1) | ||
| Invasion depth | |||||
| T1/T2 | 21 | 39 | 28.0 (16.5–39.5) | 2.025 | 0.155 |
| T3/T4 | 71 | 13 | 27.0 (21.9–32.1) | ||
| Lymph node metastasis | |||||
| No | 55 | 34 | 27.0 (17.8–36.2) | 0.177 | 0.674 |
| Yes | 37 | 18 | 28.0 (24.6–31.4) | ||
| TNM stage | |||||
| I+II | 32 | 15 | 40.0 (35.7–44.3) | 6.047 | 0.014 |
| III+IV | 60 | 37 | 19.0 (14.4–23.6) | ||
| 15-PGDH expression | |||||
| High | 58 | 30 | 40.0 (30.2–49.8) | 7.195 | 0.007 |
| Low | 34 | 22 | 19.0 (17.9–20.1) | ||
| FOXP3 expression | |||||
| Low | 55 | 29 | 40.0 (35.3–44.7) | 6.844 | 0.009 |
| High | 37 | 23 | 25.0 (18.5–31.5) |
Abbreviations: T2, tumour invasion of the muscularis propria or subserosa; T3, tumour invasion extends to or beyond the serosa; T4, tumour invasion of adjacent structures; Tubular, tubular adenocarcinoma; Poor, poorly differentiated adenocarcinoma.
Table 4.
Predictive Factors for Overall Survival by Multivariate Analysis in GC (Single Space)
| B | Wald | P-value | Hazard Ratio, 95% CI | |
|---|---|---|---|---|
| Differentiation | ||||
| Tubular | −0.176 | 0.295 | 0.587 | 0.838 (0.444–1.584) |
| Poorly | ||||
| Invasion depth | ||||
| T2 | −0.323 | 0.673 | 0.412 | 0.724 (0.335–1.566) |
| T3/T4 | ||||
| Lymph node metastasis | ||||
| No | −0.526 | 2.805 | 0.094 | 0.591 (0.319–1.094) |
| Yes | ||||
| 15-PGDH expression | ||||
| High | −0.503 | 9.511 | 0.002 | 0.605 (0.440–0.833) |
| Low | ||||
| FOXP3 expression | ||||
| Low | 0.496 | 2.416 | 0.120 | 1.641 (0.879–3.066) |
| High |
Abbreviations: T2, tumour invasion of the muscularis propria or subserosa; T3, tumour invasion extends to or beyond the serosa; T4, tumour invasion of adjacent structures; Tubular, tubular adenocarcinoma; Poor, poorly differentiated adenocarcinoma.
Discussion
In the current study, we observed that expression of 15-PGDH was significantly lower in GC than in matched non-GC gastric tissue, and showed that the lower level of 15-PGDH expression in GC patients was significantly associated with poor differentiation and lymph node metastasis. Importantly, 15-PGDH expression, as analyzed by multivariate analysis, may represent an independent predictor of improved prognosis in GC patients.
The observation that gastric 15-PGDH expression is significantly lower in GC than in non-GC gastric tissue, coupled with its direct correlation with GC differentiation as well as lymph node metastasis, suggest that 15-PGDH plays a role maintaining homeostasis of the gastric mucosa in the normal gastrointestinal micro-environment. Our data further suggest that a low level of 15-PGDH expression in GC promotes tumor growth and spread. This concept is consistent with findings of Tatsuwaki et al7 and Liu et al,19 showing that 15-PGDH is an anti-inflammatory cytokine that contributes to GC progression.
Inflammatory responses are considered part of the multi-step process, and one of the many factors involved, in GC development;20 among these, the cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) pathway was first identified as a key player in tumorigenesis and rapid growth and metastasis of GC. The anti-inflammatory role of 15-PGDH has been previously demonstrated. Here, we found that reduced expression of 15-PGDH in GC is consistent with dysregulated gastric mucosal immunity in the micro-environment during the development of GC.
The immunosuppression of tumor cells is deemed to play a vital role in tumorigenesis and thus invasion and metastasis, and is dependent on the density and type of tumor-infiltrating lymphocytes (TILs).11 CD4+/CD25+ Treg cells play a major role in immune escape and suppression of the anti-tumor immune response, thus promoting tumor growth and invasion.12,13 The study of tumor immunosuppression caused by increased numbers of Treg cells in the tumor stroma is emerging as a topic of much interest in tumor research.14 It has been reported that in numerous cancers, such as lung cancer, gastric cancer, intestinal cancer, and liver cancer, Tregs accumulate in peritumorous tissues at levels higher than those in the normal control group, and that such accumulation is indicative of poor prognosis.15
The binding of PGE2 receptors on the surface of Treg cells with their ligands induces FOXP3 expression and enables T cells to acquire inhibitory activity.17 The COX-2/PGE2 signaling pathway increases Treg FOXP3 expression in GC and is associated with poor prognosis.21 Although the precise mechanisms involved in the production and activation of neoplastic Treg cells are still unclear, these may be related to the production of cytokines, such as PGE2 and TGF-beta, by tumor cells, which can induce the production of Tregs.22,23 PGE2 is one of the main factors implicated in abnormal levels of increase in Treg cells. However, 15-PGDH catalyzes the oxidation of PGE2, leading to its degradation and inactivation. In the current study, we explored the correlation between the expression of 15-PGDH and that of FOXP3, and found that as 15-PGDH expression decreased, that of FOXP3 increased, leading to the accumulation of FOXP3+ Tregs in tumor stroma.
In the present study, the survival curve demonstrated that GC patients with higher 15-PGDH expression had better prognosis and longer survival. Our data also showed that the level of 15-PGDH expression is an important determinant of survival of patients with GC. Furthermore, in two sub-groups with GC (patients with high FOXP3 expression and patients who were TNM III–IV at surgery), high levels of 15-PGDH expression resulted in a significant survival benefit, suggesting that 15-PGDH seems to be a reliable and consistent independent factor in predicting the prognosis of GC.
In this study, we only examined 15-PGDH expression within GC and adjacent non-cancer tissue at the time of surgery. It is still not clear whether reduced 15-PGDH expression within normal gastric tissue renders the stomach more susceptible to the development of GC; this unresolved point will be investigated in our future studies. More importantly, the mechanism by which 15-PGDH influences anti-tumor immunity via FOXP3 expression in Tregs cells constitutes another important point for future study. In addition, we acknowledge that the sample size in the current study was not large, and future research should aim to carry out multi-center studies with larger numbers of patients. The Department of Pathology at the Gansu University of Chinese Medicine mainly focuses on teaching, and our “tissue bank” is therefore rather small. The current experiments thus represent proof of concept. Studies with larger sample sizes and examining additional signaling pathways, with the aim of identifying novel therapeutic targets, are now warranted.
Conclusion
The present study suggests that, based on multivariate analysis, 15-PGDH represents a reliable predictive factor for the prognosis of GC, constituting a strong and reliable predictor of survival post-surgery. Furthermore, our data suggest that 15-PGDH may contribute to anti-tumor immunity. The precise involvement of FOXP3+ Treg cells in mediating anti-tumor effects will be determined in our future studies via flow cytometry and ex-vivo experiments.
Acknowledgments
The authors thank Professor Bao Bob (Sydney University) for providing assistance with language polishing.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 81960869); the Foundation of Key Laboratory of Dunhuang Medicine and Transformation Constructed by Chinese Ministry of Education and Gansu Province (No. DHYX18-12); and the Youth Research Foundation of the Gansu University of Chinese Medicine (No. ZQ2017-2).
Abbreviations
15-PGDH, 15-hydroprostaglandin dehydrogenase; GC, gastric cancer; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; Treg, regulatory T; TILs, tumor-infiltrating lymphocytes; OS, overall survival; FOXP3, forkhead box protein 3; IHC, immunohistochemical.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
The study was approved by the Medical Ethics Committee of Gansu Wuwei Tumor Hospital (ethical approval was provided on March 5th, 2019).
Author Contributions
YLL, DLL, JZH, and YQL performed the conception, design and acquisition of data; JJL, JJD, LZ, YLS and CXM performed the analysis and interpretation of data; All authors contributed to drafting the article or revising the manuscript critically for important intellectual content; All authors approved the final version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Oshima H, Oshima M. The role of PGE2-associated inflammatory responses in gastric cancer development. Semin Immunopathol. 2013;35:139–150. doi: 10.1007/s00281-012-0353-5 [DOI] [PubMed] [Google Scholar]
- 4.Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett. 2010;295:7–16. doi: 10.1016/j.canlet.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 5.Jang TJ, Ji YS, Jung KH. Decreased expression of 15-hydroxyprostaglandin dehydrogenase in gastric carcinomas. Yonsei Med J. 2008;49:917–922. doi: 10.3349/ymj.2008.49.6.917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song HJ, Myung SJ, Kim IW, et al. 15-hydroxyprostaglandin dehydrogenase is downregulated and exhibits tumoursuppressor activity in gastric cancer. Cancer Invest. 2011;29:257–265. doi: 10.3109/07357907.2011.568562 [DOI] [PubMed] [Google Scholar]
- 7.Tatsuwaki H, Tanigawa T, Watanabe T, et al. Reduction of 15-hydroxyprostaglandin dehydrogenase expression is an independent predictor of poor survival associated with enhanced cell proliferation in gastric adenocarcinoma. Cancer Sci. 2010;101:550–558. doi: 10.1111/j.1349-7006.2009.01390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo NJ, Jeong EG, Lee SH, Lee SH. Expression of 15-hydroxyprostaglandin dehydrogenase, a COX-2 antagonist and tumour suppressor, is not altered in gastric carcinomas. Pathology. 2007;39:174–175. doi: 10.1080/00313020601123946 [DOI] [PubMed] [Google Scholar]
- 9.Thiel A, Ganesan A, Mrena J, et al. 15-hydroxyprostaglandin dehydrogenase is down-regulated in gastric cancer. Clin Cancer Res. 2009;15:4572–4580. doi: 10.1158/1078-0432.CCR-08-2518 [DOI] [PubMed] [Google Scholar]
- 10.Quezada SA, Peggs KS. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br J Cancer. 2013;108(8):1560–1565. doi: 10.1038/bjc.2013.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99(10):1704–1711. doi: 10.1038/sj.bjc.6604738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012;22(4):327–334. doi: 10.1016/j.semcancer.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteside TL, Schuler P, Schilling B. Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther. 2012;12(10):1383–1397. doi: 10.1517/14712598.2012.707184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol. 2013;34(1):33–40. doi: 10.1016/j.it.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee A, Vasanthakumar A, Grigoriadis G. Modulating T regulatory cells in cancer: how close are we? Immunol Cell Biol. 2013;91(5):340–349. doi: 10.1038/icb.2013.12 [DOI] [PubMed] [Google Scholar]
- 16.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan XL, Chen L, Li MX, et al. Elevated expression of FOXP3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134(3):277–288. doi: 10.1016/j.clim.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 18.Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2016:203–220. [Google Scholar]
- 19.Liu Z, Wang X, Lu Y, et al. Expression of 15-PGDH is downregulated by COX-2 in gastric cancer. Carcinogenesis. 2008;29:1219–1227. doi: 10.1093/carcin/bgm297 [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 21.Lee SW, Kim YM, Lee HY, et al. Proliferation of CD4CD25FOXP3 regulatory T lymphocytes in ex vivo expanded ascitic fluid from primary and recurrent ovarian carcinoma. J Gynecol Oncol. 2010;21(1):38–44. doi: 10.3802/jgo.2010.21.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimura A, Muto G. TGF-β function in immune suppression. Curr Top Microbiol Immunol. 2011;350:127–147. doi: 10.1007/82_2010_87 [DOI] [PubMed] [Google Scholar]
- 23.Ma X, Holt D, Kundu N, et al. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology. 2013;2(1):e22647. doi: 10.4161/onci.22647 [DOI] [PMC free article] [PubMed] [Google Scholar]