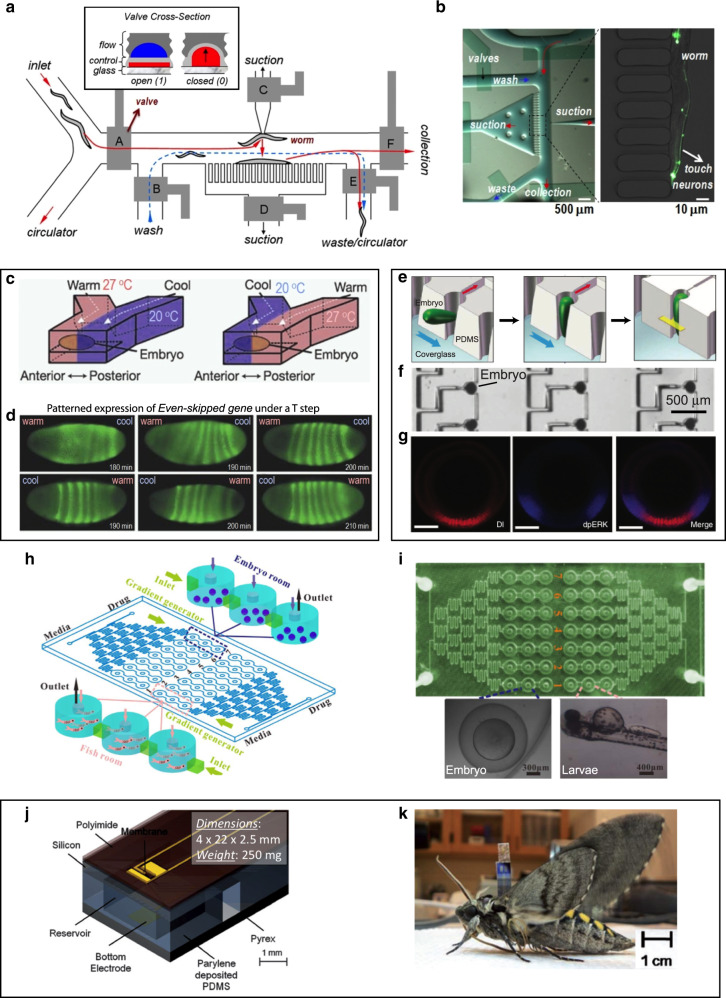
Fig. 6. Microfluidic devices for small-animal research.
a, b Microfluidic worm sorter. a Microfluidic worm-sorter schematics. The sorter consists of control channels and valves (gray) that direct the flow of worms in the flow channels in different directions with valves (labeled A–F) in order to isolate and capture individual worms, image them, and finally sort to waste or collection. b Micrograph of the device during image acquisition from a trapped worm. Adapted with permission from ref. 147. c, d PDMS microfluidic device for D. melanogaster embryo development in response to a temperature step. c Schematic of the experimental setup. d Fluorescence micrographs of D. melanogaster gene expression after exposure to a temperature step that was applied before the first image. Remarkably, over time the embryos exposed to inverse steps ended up expressing the same (correct) patterns. Adapted with permission from ref. 153. e–g A microfluidic array for large-scale trapping of Drosophila embryos. e Schematic showing the embryo trapping process: the flow (blue arrows) guides the embryo into the trap and orients it vertically; finally, the trap contracts and secures the embryo. f Micrograph showing a section of the array with trapped embryos. g Confocal images of signal transduction and morphogen gradients in dorsoventral patterning activated by Dorsal (Dl, an NF-κB transcription factor, which subdivides the embryo into three germ layers) and phospho-MAPK (dpERK). Adapted with permission from ref. 154. h, i A microfluidic platform for real-time monitoring of drug-induced developmental toxicity in zebrafish. h Schematics of the zebrafish platform, which includes two independent zones, each with a media inlet, a drug inlet, a gradient generator and seven series of fish tanks (each concentration with three tanks). i Photo of the microfluidic chip (top) and micrographs (bottom) of an embryo (left) and larvae (right) in the chip. Adapted with permission from ref. 156. j, k Microfluidics for engineering insect flight metabolics, implanted at an immature stage. j Schematic of the electroactive microwell drug delivery system. kM. sexta moth successfully emerged from the pupa with an implanted device. Adapted with permission from ref. 164