Figure 2.
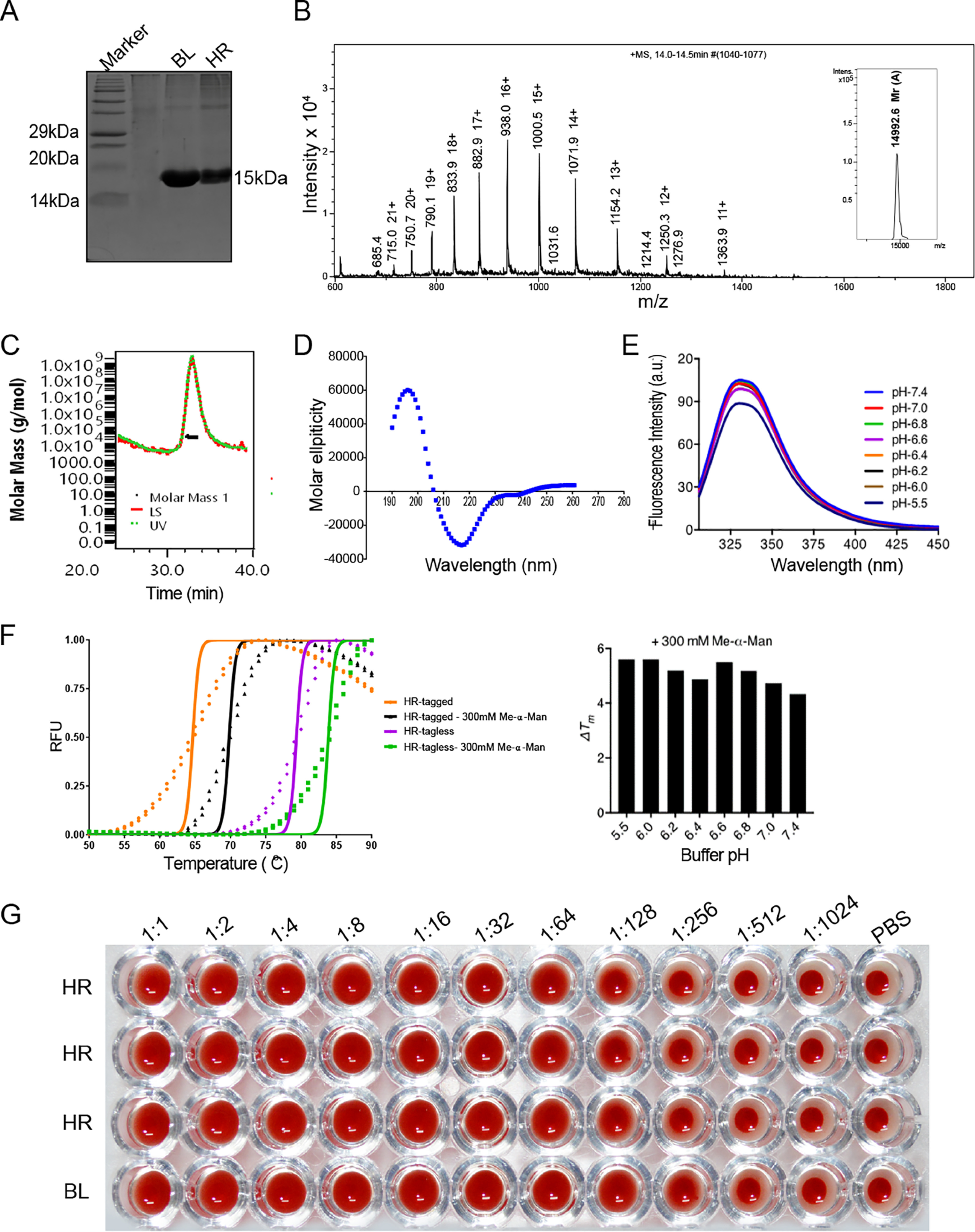
Protein expression, purification, and in vitro characterization of horcolin. A, 15% SDS-PAGE showing purity of the recombinant horcolin protein subsequent to purification with affinity chromatography. Lane 1, marker; lane 2, HR; lane 3, BanLec. HR appears as a single band with Mr ∼15 kDa in SDS–PAGE. B, spectrum of intensity versus mass and charge ratio, showing the observed experimental mass of the purified protein from electrospray ionization-MS (14,992 Da). C, SEC-MALS profile for the HR protein. The peak at 30 ml corresponds to Mr ∼28.9 kDa (D) far-UV CD spectra of the purified recombinant HR showing β-sheet composition. E, intrinsic fluorescence emission spectra of protein across pH range 5.5–7.4. F, thermal shift assay of horcolin (tagged and tagless) at varying pH conditions along with the ligand-induced stabilization (Me-α-Man) to determine the effect of pH on protein thermal stability. G, an initial concentration of 25 μg/ml was serially diluted (2-fold) to characterize the hemagglutination activity of the lectin. The experiment was done as triplicates for HR with BanLec (BL) as the positive control and 1× PBS as the negative control.
