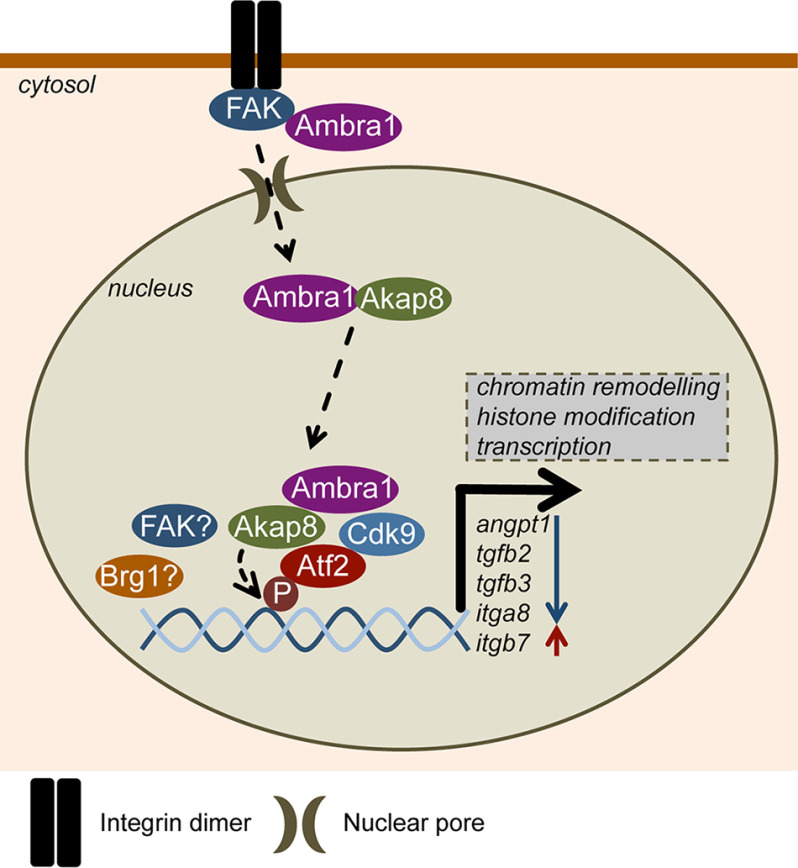
Figure 7.
Model depicting Ambra1-dependent transcriptional regulation. In mouse SCC cells, Ambra1 is already known to localize to autophagosomes and focal adhesions, where it binds FAK and Src and regulates the removal of untethered kinases via autophagy. Ambra1 can also interact with nuclear pore components and is translocated into the nucleus most likely via nuclear pores and importins. Nuclear Ambra1 is part of a network consisting of chromatin modifiers and transcriptional regulators, some of which are recruited to chromatin in an Ambra1-mediated manner, including the PKA scaffold Akap8, Cdk9, and active Atf2 (p-Atf2 T71). Further, Ambra1, Akap8, Cdk9, and Atf2 coregulate the expression of a subset of genes, like Angpt1, Tgfb2, Tgfb3, Itga8, and Itgb7. Both Ambra1 and Akap8 influence cellular histone modifications, which could contribute to their transcriptional effects. Overall, the autophagy protein Ambra1 also acts as a nuclear platform to recruit key scaffolds, chromatin modifiers, and transcriptional regulators to elicit gene expression changes via Atf2.