Abstract
Background
Vancomycin is a common first-line option for MRSA infections. The heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) phenotype is associated with therapeutic failure. However, hVISA isolates are usually reported as vancomycin susceptible by routine susceptibility testing procedures.
Objectives
To detect and characterize the hVISA phenotype in MRSA isolates causing infections in nine Latin American countries.
Methods
We evaluated a total of 1189 vancomycin-susceptible MRSA isolates recovered during 2006–08 and 2011–14. After an initial screening of hVISA using glycopeptide-supplemented agar strategies, the detection of hVISA was performed by Etest (GRD) and Macro-method (MET). Isolates deemed to be hVISA were subjected to population analysis profile/AUC (PAP/AUC) and WGS for further characterization. Finally, we interrogated alterations in predicted proteins associated with the development of the VISA phenotype in both hVISA and vancomycin-susceptible S. aureus (VSSA) genomes.
Results
A total of 39 MRSA isolates (3.3%) were classified as hVISA (1.4% and 5.6% in MRSA recovered from 2006–08 and 2011–14, respectively). Most of the hVISA strains (95%) belonged to clonal complex (CC) 5. Only 6/39 hVISA isolates were categorized as hVISA by PAP/AUC, with 6 other isolates close (0.87–0.89) to the cut-off (0.9). The majority of the 39 hVISA isolates exhibited the Leu-14→Ile (90%) and VraT Glu-156→Gly (90%) amino acid substitutions in WalK. Additionally, we identified 10 substitutions present only in hVISA isolates, involving WalK, VraS, RpoB and RpoC proteins.
Conclusions
The hVISA phenotype exhibits low frequency in Latin America. Amino acid substitutions in proteins involved in cell envelope homeostasis and RNA synthesis were commonly identified. Our results suggest that Etest-based methods are an important alternative for the detection of hVISA clinical isolates.
Introduction
MRSA is a major human pathogen causing a broad spectrum of diseases ranging from mild skin infections to life-threatening bacteraemia.1 The emergence and dissemination of MRSA are considered to be serious public health concerns. Recent data on the prevalence of Staphylococcus aureus bacteraemia in nine Latin American countries showed a highly heterogeneous prevalence of MRSA ranging from 22% to 62%.2 Glycopeptides, particularly vancomycin, have been the treatment of choice for MRSA infections for decades. Unfortunately, the increasing rates of MRSA infections and excessive use of vancomycin might have influenced the emergence of bacterial isolates with reduced susceptibility to this antibiotic.3
Decreased susceptibility to vancomycin was first identified in 1997, leading to therapeutic failure.4,5 In 2002, the first S. aureus isolate with high-level resistance to vancomycin (VRSA) was reported in the USA and since then other cases have been described, mostly in the USA6 and Brazil.7,8 The VRSA phenotype is due to acquisition of the vanA cluster,9,10 which is readily detected by conventional MIC methods.11 In contrast, the mechanisms responsible for vancomycin-intermediate S. aureus (VISA) are complex and multifactorial, converging in a phenotype that is associated with thickening of the cell wall. Some of the most frequent genetic changes in VISA isolates are found in genes encoding cell envelope regulatory systems (vraTSR, graSR and walKR), membrane-associated (tcaA) and capsular polysaccharide (capP) proteins and those encoding the β subunit of the RNA polymerase (rpoB and rpoC).12,13 Other phenotypic alterations observed in VISA strains include slow growth rate, high capsular expression, reduced autolysis and low peptidoglycan cross-linking, among others.14 Of note, some of these phenotypic changes, particularly those associated with cell-wall thickening and changes in cell-wall electric charge,15 may also affect other classes of antibiotics such as the lipopeptide daptomycin.16
Another distinct phenotype found in clinical isolates is the heterogeneous VISA (hVISA) phenotype. These isolates are classified as susceptible (vancomycin MICs ≤2 mg/L) through standard methodologies.11 hVISA isolates contain cellular subpopulations (105 to 106 cfu/mL) with a vancomycin MIC within the intermediate range (4–8 mg/L) that are not detected by standard susceptibility methods such as automated methods, broth microdilution or Etest.14 Due to the lack of detection of hVISA, its clinical impact remains poorly understood. However, studies have shown that infections caused by hVISA strains enhance the risk of therapeutic failure and carry higher hospital costs due to longer hospital stays compared with subjects infected with vancomycin-susceptible S. aureus strains (VSSA).17–19
Different methodologies have been proposed for detection of hVISA. Agar supplemented with glycopeptides (vancomycin or teicoplanin) may result in many false positives due to low specificity (53%–65%).14 Additionally, Etest-based approaches, including the glycopeptide resistance detection (GRD) test and Etest macro method (MET) could facilitate detection of hVISA. However, these are not recommended by CLSI or EUCAST and are not usually implemented as routine techniques in clinical laboratories.11,20,21 Currently, the most reliable method for hVISA detection is population analysis profile/AUC (PAP/AUC). This method detects and quantifies subpopulations able to grow at higher vancomycin concentrations when compared with the hVISA reference strain Mu3.14,22 Unfortunately, PAP/AUC is cumbersome and expensive and it is not suitable to be implemented in a clinical laboratory as a routine diagnostic technique.
Previous reports suggest a general prevalence of hVISA from 0.2% up to 19.5%23–25 in Latin America, although the actual prevalence of hVISA is unknown in most countries. During 2009–10, Argentina reported an hVISA prevalence of 3.3% in S. aureus causing bacteraemia in a teaching hospital.26 Further, sporadic characterization of hVISA strains has been reported in isolates recovered from Chile, Venezuela and Brazil.27–30 Here, using a large collection of bloodstream S. aureus isolates from patients in nine Latin American countries, we sought to identify the frequency of hVISA isolates. Furthermore, we developed a genomic framework to identify genetic features likely to be associated with the hVISA phenotype in these isolates.
Materials and methods
Bacterial isolates
From a total of 2755 S. aureus clinical isolates that were collected in two multicentre surveillance studies performed in Latin America (2006–08 and 2011–14), we included 1189 isolates confirmed as MRSA both phenotypically and molecularly. In the first study, 1570 S. aureus isolates causing infections in adult patients in 32 hospitals from Colombia, Ecuador, Peru and Venezuela were collected between 2006 and 2008, with 651 (41.5%) identified as MRSA.31 In the second study, 1185 S. aureus isolates were recovered from patients with bacteraemia in 24 hospitals from Argentina, Brazil, Chile, Colombia, Ecuador, Guatemala, Mexico, Peru and Venezuela.2 Of these, 538 (45%) were MRSA. Confirmation of S. aureus species and resistance to β-lactam antibiotics mediated by mecA was performed by multiplex PCR.32 Antimicrobial susceptibility profiles were determined by the agar dilution method following CLSI recommendations.11 The genetic relationships and MRSA lineages were determined by PFGE and MLST, as previously described.33,34
Screening for the VISA and hVISA phenotypes
We used brain heart infusion (BHI) agar supplemented with vancomycin 6 mg/L (BHI-V6) to screen for VRSA strains, as recommended by CLSI.11 For VISA detection, we used previously described screening approaches.14,35 Briefly, BHI agar with vancomycin 4 mg/L (BHI-V4) was inoculated with 10 μL of a 0.5 McFarland bacterial suspension. Simultaneously, Mueller–Hinton (MH) agar supplemented with teicoplanin 5 mg/L (MH-T5) was inoculated with 10 μL of bacterial suspension, using a 2.0 McFarland inoculum. Cultures were read at 48 h. A positive result was defined as the growth of ≥2 colonies on the agar.
GRD and MET methods
All isolates positive by at least one of the initial screening tests were further evaluated with the GRD strip. Positive isolates were then tested by MET. For GRD, an inoculum was prepared from an overnight culture in BHI broth. A bacterial suspension equivalent to a 0.5 McFarland standard was plated onto MH agar supplemented with 5% sheep blood and a double-ended Etest strip (bioMérieux® and Liofilchem®) with vancomycin and teicoplanin (0.5–32/0.5–32 mg/L) was applied. Plates were incubated at 37°C for 48 h.36 For MET, 200 μL of a bacterial inoculum equivalent to 2.0 McFarland was plated onto BHI agar. Vancomycin and teicoplanin Etest strips (bioMérieux®) were applied and plates were incubated at 37°C for 48 h. A positive result was defined as the presence of any growth at concentrations of ≥8 mg/L for one or both antibiotics (vancomycin and teicoplanin).36S. aureus ATCC 700698 (Mu3; hVISA), ATCC 700699 (Mu50; VISA) and ATCC 29213 (VSSA) were used as control strains.
PAP/AUC
All GRD- and MET-positive isolates were subsequently tested by PAP/AUC using the microdilution method, as described previously.22,37 In brief, a bacterial suspension equivalent to approximately 5.0 × 107 cfu/mL from an overnight culture was prepared. Serial dilutions (10−1 to 10−7) were plated on BHI agar containing increasing concentrations of vancomycin (0 to 12 mg/L). To test the isolates from the second surveillance, the methodology was modified slightly by reducing the volume of the bacterial suspension from 1 mL to 0.1 mL.37 After 48 h of incubation at 37°C, cfu were manually counted and results were determined for each vancomycin concentration and expressed in log10 cfu/mL. This process was performed in triplicate for each strain. We also determined the AUC for each strain based on the average log10 cfu/mL at each concentration of vancomycin using the trapezoidal method. The PAP/AUC ratio was calculated as AUCstrain/AUCMu3. Ratios of 0.9 to 1.3 were considered as compatible with the hVISA phenotype and those >1.3 deemed compatible with a VISA strain.22
WGS, in silico typing and phylogenetics
A total of 39 MRSA isolates categorized as hVISA by GRD and MET (n = 39) and 305 vancomycin-susceptible MRSA were sequenced. Briefly, genomic DNA was extracted from overnight cultures using the DNeasy® Blood & Tissue kit (QIAGEN) after 30 min of lysostaphin treatment at 37°C. DNA libraries were prepared using Nextera XT DNA sample preparation (Illumina®, San Diego, CA, USA), according to the manufacturer recommendations. Raw reads were trimmed at their ends if their quality was below a Phred score of 30 using Trimmomatic38 and de novo assembled using SPAdes v3.13.39 SCCmec typing was performed in silico using reported primers.40,41 The type of agr was determined using the reference sequences for agr-I (X52543.1), agr-II (AF001782.1), agr-III (AF001783.1) and agr-IV (AF288215.1). The resistome was determined using the ResFinder database42 and mutations associated with resistance to fluoroquinolones (GyrA WP_000819088.1 and GrlA WP_000255586.1), rifampicin (RpoB CAG42275.1) and linezolid (G2576T in DNA coding the 23S rRNA and changes in RplC YP_000979/RplD YP_500978) were identified using the NCBI BLASTX tool.43 Genome annotation was performed using the RAST server.44 Sequencing data of the hVISA and VSSA isolates from this study are available in NCBI BioProjects: PRJNA595928, PRJNA291213, PRJNA393041 and PRJNA247399 (see Table S1, available as Supplementary data at JAC Online).
The genetic relationship of the hVISA isolates was determined by a phylogenetic tree based on the core genome from the sequenced genomes, as well as the genome sequences of S. aureus FPR3757, N315, Mu3, Mu50 and ATCC 29213 (NCBI GenBank accession numbers: NC_007793.1, NC_002745.2, NC_009782.1, NC_002758.2 and LHUS02000001.1, respectively). Core genome was calculated with Roary.45 The nucleotide sequences of each of the orthogroups defined in the core genome were aligned using MUSCLE46 and these alignments were further concatenated to obtain a phylogenetic matrix. The matrix was used in RAxML47 version 8.2.9 to reconstruct phylogeny of these strains, with a GAMMA model of rate heterogeneity selecting the best tree from 20 different runs and 1000 bootstrap resampling. The trees were edited using the interactive tree of life (iTol) tool (https://itol.embl.de/).48
Detection of changes potentially associated with VISA phenotype
Amino acid substitutions were identified in the sequences of WalKR (Q7A8E0.1 and Q7A4R9.1), VraTSR (OBY00462.1, Q99SZ7.1 and Q7A4R9.1), GraSR (Q7A6Z3.1 and Q99VW2.1), RpoB (BAB41731.1), RpoC (BAB41732.1), TcaA (BAB43448.1) and CapP (BAB41379.1) predicted proteins, which have been associated with the VISA phenotype. Amino acid sequences were compared through multiple sequence alignments using MUSCLE.46 We included the genomes of VSSA strains ATCC 29213, N315 and FPR3757, as well as the hVISA and VISA prototypical isolates Mu3 and Mu50, respectively. An amino acid change was defined as the presence of a residue in a specific position that was different from those occurring in ATCC 29213, N315 and FPR3757. The chi-squared test was used to evaluate statistically significant differences between hVISA and VSSA isolates harbouring amino acid changes, using a significance level of 95% and the R programming language.49
Ethics
Ethics approval was obtained from the Ethics Review Committee, Universidad El Bosque (UEB 410-2016), Acta No. 012-2016.
Results
Low frequency of hVISA phenotype among clinical Latin American MRSA isolates
We defined hVISA as S. aureus isolates that were positive by both agar screening tests (BHI-V4 and/or MH-T5) and Etest-based methods (both GRD and MET). No VRSA were found in our collection using the BHI-V6 screening test. Additionally, we found that 43% of the isolates were positive using BHI-V4 and/or MH-T5. Table 1 shows the frequency of hVISA isolates in the two surveillance periods. In the initial surveillance period (2006–08), a total of 651 MRSA isolates were included and 27%, 26% and 8% were obtained from blood, surgical wounds and skin and soft tissue infections, respectively. Only 9 isolates (1.4%) were considered to be hVISA (by GRD and MET) (Table 1). In the second surveillance, which included bloodstream isolates only, (70% of which were collected from patients who received glycopeptide therapy), 30 (5.6%) MRSA were deemed to be hVISA (by GRD and MET). Interestingly, 25 of the 30 isolates (83%) were recovered from hospitals in Chile and Peru, which have a high prevalence of the clonal complex (CC)5 Chilean/Cordobes clone2 (see below). Among the 39 hVISA MRSA, the majority (34; 87.2%) were obtained from blood, while 3 (7.6%) were recovered from bronchial aspirates, 1 (2.6%) from pleural fluid and 1 (2.6%) from a surgical wound.
Table 1.
Prevalence of hVISA phenotype (detected by GRD and MET) in Latin American MRSA isolates
| Country | 2006–08a |
2011–14a |
||
|---|---|---|---|---|
| MRSA (n) | hVISA, n (%) | MRSA (n) | hVISA, n (%) | |
| Brazil | — | — | 126 | 1 (0.8) |
| Peru | 178 | 6 (3.4) | 84 | 16 (19.0) |
| Chile | — | — | 74 | 9 (12.2) |
| Guatemala | — | — | 74 | ND |
| Argentina | — | — | 60 | 3 (5.0) |
| Colombia | 318 | 2 (0.6) | 41 | ND |
| Venezuela | 69 | — | 33 | ND |
| Ecuador | 86 | 1 (1.2) | 29 | 1 (3.4) |
| Mexico | — | — | 17 | ND |
| Total | 651 | 9 (1.4) | 538 | 30 (5.6) |
ND, not determined.
Period of time of multicentre study surveillance.
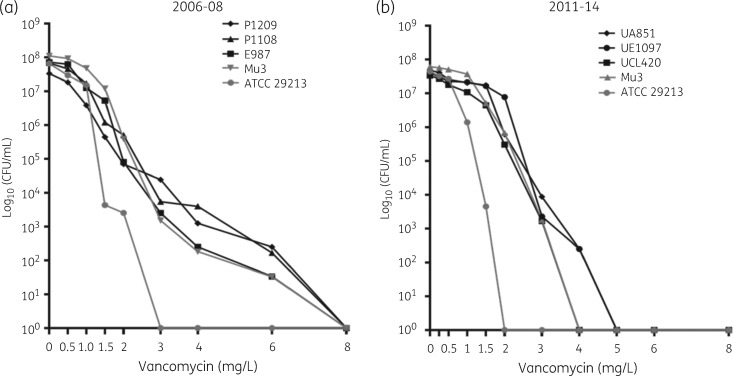
The 39 hVISA isolates were subsequently examined by the PAP/AUC methodology.22,37 Using this approach, only 6 (0.5%) isolates were confirmed as hVISA (PAP/AUC ratios between 1.03 and 1.19) (Figure 1). Of note, 6 (0.5%) other isolates exhibited a ratio between 0.87 and 0.89 when compared with Mu3, a value close to the lower limit of detection for hVISA (0.9) (Figure 2). These latter isolates exhibited high cfu counts (>1 × 106 cfu/mL) at a vancomycin concentration of 1.5 mg/L (data not shown).
Figure 1.
PAP of isolates confirmed as hVISA by PAP/AUC using vancomycin. cfu determinations at increasing vancomycin concentrations are shown. Results are representative of three independent experiments. Strains from the study with AUC ratio higher than 0.9 when compared with the AUC of S. aureus Mu3 are shown in black. Control strains of hVISA (ATCC 700698; Mu3) and VSSA (ATCC 29213) are represented by triangles and grey circles, respectively. Panels (a) and (b) include the results of strains collected during 2006–08 and 2011–14, respectively, which were performed by different methodologies.
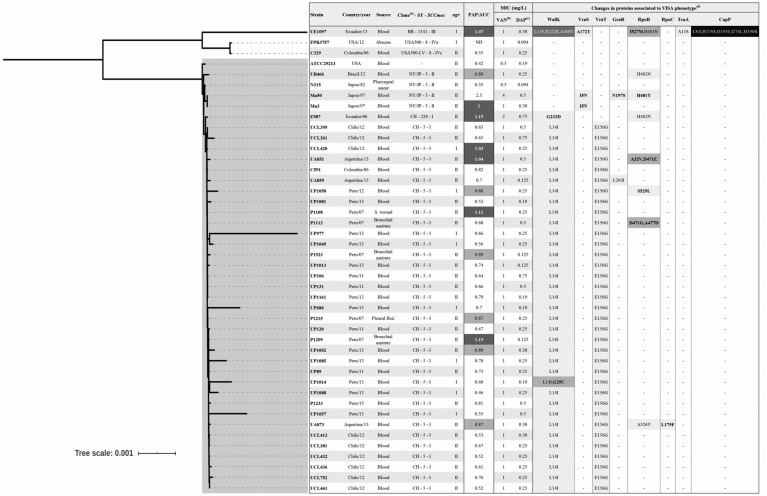
Figure 2.
Phylogenetic relationships and changes in proteins associated with the hVISA phenotype. The genetic relationship was determined by SNP-based phylogenetic tree using the core genome. The clade of isolates belonging to CC5 is highlighted in grey. aBR (Brazilian clone); CH (Chilean/Cordobes clone); NY/JP (New York/Japan clone). bPAP/AUC ratio values higher than 0.9 when compared with the Mu3 strain are highlighted in dark grey and isolates with ratio values between 0.87 and 0.89 in light grey. cVancomycin MICs (mg/L) were determined by the agar dilution method. dDaptomycin MICs (mg/L) were determined by the Etest method. eShade intensity is proportional to the accumulation of changes in the predicted protein. Changes in bold were not present in VSSA isolates.
MRSA isolates exhibiting the hVISA phenotype belong to CC5 lineage
In Latin American hospitals, there is a wide diversity of MRSA genetic lineages circulating and clonal lineages CC5, CC8 and CC30 are the most prevalent.2,3 Among the 39 hVISA, 95% belonged to CC5, showing PFGE profiles related to Chilean/Cordobes (SCCmecI, ST5/228) and New York/Japan clones (NY/JP-SCCmecII; ST5; 1 isolate). Further, among the hVISA isolates belonging to the CC5 Chilean/Cordobes clone, 35 isolates (97%) were ST5 and 1 isolate (3%) was ST228. Of note, only one hVISA isolate belonged to CC8, which was related to the USA300 Latin American variant (USA300-LV) (SCCmec-IVc, ST-8) harbouring agr-II. The other isolate belonged to CC239 (Brazilian clone; SCCmec-III, ST-1341, a single-locus variant of ST239), harbouring agr-I. The core genome-based phylogenetic reconstruction confirmed a genetic relationship among isolates belonging to CC5 (Figure 2). Of note, most of the hVISA isolates that had a PAP/AUC above the threshold (5/6) were not phylogenetically related although they belonged to CC5 (Figure 2).
Phenotypic resistance profiles indicated that the 39 hVISA isolates exhibited resistance to erythromycin, clindamycin, ciprofloxacin and gentamicin. Only 18% were resistant to chloramphenicol, 8% to tetracycline, 5% to rifampicin and 3% to trimethoprim/sulfamethoxazole (Table S2). None of the isolates were resistant to vancomycin, teicoplanin, linezolid, minocycline or daptomycin (daptomycin MIC90 of 0.5 mg/L). The resistome analysis is shown in Table S3. Most (82%) of the hVISA isolates carried blaZ and genes encoding aminoglycoside-modifying enzymes: ant(9)-Ia (97.4%); ant(6)-Ia (84.6%); and aph(3′)-III (84.6%). The erm(A) gene conferring macrolide, lincosamide and streptogramin B (MLSB) resistance was present in 38 isolates (97.4%), while erm(C) was only identified in 2 isolates (5.1%). Changes associated with fluoroquinolone resistance in the putative GrlA and GyrA proteins were identified in the majority of the hVISA isolates (38/39; 97.4%). Of note, 16% of hVISA exhibited changes in RpoB associated with resistance to rifampicin. We also identified tet(M) and fosD in two isolates [one with tet(M) and the other with fosD].
Changes in components of WalKR and VraTRS systems potentially associated with hVISA phenotype in Latin American MRSA isolates
Next, we used the genomic information to interrogate changes in predicted proteins that have been previously postulated to contribute to the development of the VISA phenotype (including WalKR, VraTSR, GraSR, RpoB, RpoC, TcaA and CapP12,13) in the 39 hVISA isolate genomes (Figure 2). Overall, we did not observe a differential pattern of changes among the isolates confirmed by PAP/AUC. It was noticeable that the isolate UE1097 (PAP/AUC positive) exhibited the highest number of changes; however, this result could be influenced by the fact that the isolate belongs to a distant genetic lineage (non-CC5).
In order to identify the variations, the protein sequences from hVISA (n = 39) and VSSA (n = 305) isolates from our study were compared with those from VSSA reference genomes ATCC 29213, FPR3757 and N315. The majority of the hVISA isolates (95%; 37 out of 39) exhibited changes in WalK, with a Leu-14→Ile substitution present in 35 isolates. WalKR is a regulatory system involved in cell-wall homeostasis. Similarly, we identified a Glu-156→Gly substitution in VraT in 90% of the hVISA isolates. VraTSR is homologous to the LiaFSR systems in other Gram-positive organisms, functioning as a three-component regulatory system that orchestrates the cell envelope stress response.50 Of note, an additional isolate exhibited a change in VraS (Ala-172→Thr). Further, seven isolates exhibited changes in RpoB, including the His-481→Tyr substitution (observed in three isolates) that has been previously identified in Mu50.51,52 Among the six isolates confirmed by PAP/AUC, all but one harboured the WalK change Leu-14→Ile/Phe. However, this unique isolate had another predicted substitution in WalK (Gly-233→Asp).
Finally, we sought to determine the frequency of the above amino acid changes in predicted proteins from 305 clinical isolates of MRSA (recovered from the multicentre study performed during 2011–14)2 that were phenotypically non-hVISA by the two agar screening tests (BHI-V4 and MH-T5) (Table S3). Interestingly, both substitutions Leu-14→Ile and Glu-156→Gly in WalK and VraT, respectively, were found in 21% of the VSSA (63/305). In contrast, these changes were present in 90% (35/39) and 87% (34/39) of the hVISA isolates, respectively (P < 0.01). Additionally, other changes described above in WalK, VraS, RpoB and RpoC were exclusively present in the hVISA genomes (bold in Figure 2). Furthermore, we did not find significant differences in the presence of changes detected in the 6 hVISA PAP/AUC-positive (>0.9) isolates compared with those present in the 33 hVISA but PAP/AUC negative (<0.9) isolates (Table S4). When we compared the substitutions present in the PAP/AUC-positive isolates with those present in the 305 VSSA isolates (Table S5) plus the PAP/AUC-negative isolates (Table S6), the presence of changes in RpoB, VraS, Walk and VraT was still significantly higher in the PAP/AUC-positive isolates.
Discussion
To our knowledge, this is the first multicentre study attempting to evaluate the frequency of the hVISA phenotype in clinical isolates of MRSA from Latin American hospitals with temporal sampling (2006–08 and 2011–14). Our results show that the frequency of the hVISA phenotype (determined by GRD and MET) was low (3.3%). A previous meta-analysis by Zhang et al.23 estimated a global hVISA prevalence of around 6%. Of note, hVISA prevalence ranged from 5.6% to 6.81% in different regions of the world.23 In South America, previous reports suggested that the prevalence of hVISA in Brazil and Argentina approached 9.7%53 and 3.3%,26 respectively. Since there are no standardized laboratory tests for detecting this phenotype, these results should be interpreted with caution. Additionally, several factors seem to affect the hVISA phenotype, including storage time and source of the isolates, among others.23,24,54 In our study, we used a hierarchical approach to determine the phenotype and the results were supported by genomic information. Therefore, our findings suggest that the frequency of the hVISA phenotype among Latin American MRSA isolates seems to be low.
When analysing the genetic background of the hVISA isolates in our study, 95% belonged to CC5, (SCCmecI-ST5 and SCCmecII-ST5). Indeed, ST5 and ST239 are hospital-associated MRSA (HA-MRSA) lineages prevalent in Asia, South America and Eastern Europe and have been considered the most epidemic genotypes of hVISA/VISA strains.23 Furthermore, the Chilean/Cordobes clone had a prevalence greater than 90% in countries such as Chile and Peru,2,31 where a relatively high proportion of isolates exhibited the hVISA phenotype (12.2% and 19%, respectively). In contrast, only one hVISA isolate recovered in Colombia (2006–08) belonged to the USA300-LV clone, a unique genetic lineage circulating in that country.2 Moreover, hVISA isolates in our study showed resistance to macrolides, lincosamides, fluoroquinolones and aminoglycosides (erythromycin, clindamycin, ciprofloxacin and gentamicin), a phenotype that is also associated with Latin American CC5 MRSA,2 whereas tetracycline and trimethoprim/sulfamethoxazole resistance were 5.1% and 2.5%, respectively. Thus, our results support the notion that the genetic backgrounds of CC5 and ST239 may be prone to develop the hVISA phenotype, although it can occur in other MRSA lineages.
Interestingly, the majority of the hVISA isolates detected by Etest-based methods were negative by the PAP/AUC (only 6/39 were positive). Of note, an additional six isolates exhibited a PAP/AUC ratio very close to the hVISA definition. Moreover, we were able to detect alterations in proteins overrepresented in hVISA isolates (when compared with VSSA isolates), regardless of the PAP/AUC result. Since the PAP/AUC ratio is strictly based on the heterogeneous subpopulations of S. aureus Mu3, this technique might not detect some hVISA strains harbouring smaller subpopulations than those contained in the Mu3 strain. Considering that PAP is not an easy test to perform in clinical laboratories, our results suggest that Etest-based methods are an important alternative for the detection of hVISA clinical isolates in a faster and more reliable manner.
Our findings were supported by genomic analyses. Indeed, we found changes in amino acid sequences of predicted proteins previously associated with the VISA phenotype, such as changes in WalK (Arg-222→Lys), GraS (Leu-26→Phe, Ile-59→Leu and Thr-224→Ile) and RpoB (His-481→Asn).14,15 However, these changes were not consistently found in all of our hVISA isolates, reflecting the complex and polygenic nature of this phenotype.14,15 Of note, it has been shown that mutations in walK correlate with thickening of the cell wall, slow growth and decreased autolysis, which are phenotypic characteristics of hVISA isolates.55 Indeed, the majority of hVISA isolates evaluated in this study exhibited WalK (Leu-14→Ile) and VraT (Glu-156→Gly) substitutions not previously reported. WalKR also controls autolytic activity, particularly of two S. aureus autolysins (Atl and LytM). Furthermore, WalKR also regulates the transcription of 13 genes involved in the metabolism and degradation of the cell wall.56 Thus, our results are aligned with previous reports demonstrating heterogeneity of genetic patterns of hVISA isolates. Interestingly, a recent study by Liu et al.57 evaluated mutations involved in the development of antibiotic tolerance and resistance and found that early alterations in RpoB and RpoC (associated with rifampicin resistance) played a potential role in the vancomycin/daptomycin-tolerant phenotype. Altogether, the data support the notion that RpoB and RpoC may be implicated in the adaptive cellular changes in S. aureus for the establishment of tolerance and heteroresistance.
In summary, our comprehensive study of MRSA clinical isolates in Latin America suggests that the overall prevalence of hVISA is low, but CC5 genetic lineages circulating in particular countries of the region may be predisposed to develop this phenotype. The difficulty in the epidemiological and clinical characterization of this phenotype makes it necessary to continue with prospective and molecular surveillance studies and detect the clinical impact in severe S. aureus infections.
Supplementary Material
Acknowledgements
The following hospitals participated in the collection of isolates. Colombia: Bogotá: Fundación Salud Bosque, Hospital Simón Bolívar, Clínica de Occidente, Clínica del Niño, Clínica Shaio, Hospital El Tunal, Hospital Santa Clara, Hospital Occidente de Kennedy, Hospital Universitario Clínica San Rafael, Clínica San Pedro Claver, Hospital San Ignacio, Fundación Santa Fe de Bogotá, Clínica Infantil Colsubsidio, Clínica Saludcoop Jorge Piñeros Corpas and Instituto Nacional de Cancerología; Cali: Centro Médico Imbanaco, Clínica Saludcoop Occidente Cali and Hospital Universitario del Valle; Medellín: Hospital Pablo Tobón Uribe and Clínica Saludcoop Juan Luis Londoño; Bucaramanga: Clínica La Foscal and Fundación Cardiovascular; Neiva: Hospital Universitario Hernando Moncaleano; Pereira: Hospital Universitario San Jorge; Ecuador: Hospital Vozandes, Hospital Eugenio Espejo, Hospital Baca Ortiz, Hospital Carlos Andrade Marín, Hospital General de las Fuerzas Armadas and Hospital Carlos Andrade Marín; Perú: Hospital Guillermo Almenara, Hospital Alberto Sabogal Laboratorio Clínico Carlos Carrillo, Hospital Nacional Sergio Bernales, Instituto Nacional de Enfermedades Neoplásicas and Hospital Nacional Hipólito Unanue; Venezuela: Clínica La Floresta, Centro Medico de Caracas and Hospital Vargas de Caracas; Argentina: Buenos Aires: Hospital de Clínicas, Hospital Británico and Sanatorio Mater Dei; Brazil: São Paulo: Hospital do Servidor Publico Estadual de São Paulo; Chile: Santiago: Escuela de Medicina, Pontificia Universidad Católica de Chile and Hospital Sótero del Río; Concepción: Hospital Guillermo Grant Benavente; Guatemala, Hospital Roosevelt; and Mexico: Hospital Civil de Guadalajara, Centro Universitario Ciencias de la Salud and Universidad de Guadalajara.
Partial results of this study were presented in IDWeek2019, Washington D.C., USA, 2019; and at ECCMID 2019 in Amsterdam.
Funding
This work was supported by Departamento Administrativo de Ciencia, Tecnología e Innovación COLCIENCIAS (COL130874455850) to L.D. and Universidad El Bosque, PCI 9510–2017 to L.P.C. C.A.A. is supported by NIH/NIAID grants R01 AI134637, R21 AI143229, and K24 AI121296, UTHealth Presidential Award and University of Texas System STARS Award. J.M.M. is supported by grant FONDECYT regular Project No. 1171805, Ministry of Education, Government of Chile and the Millennium Science Initiative, Ministry of Economy, Development and Tourism, Chile.
Transparency declarations
C.A.A. has received grant support from Merck, Inc, Entasis pharmaceuticals and MeMed diagnostics. All other authors: none to declare.
Supplementary data
Tables S1 to S6 are available as Supplementary data at JAC Online.
References
- 1. Stryjewski ME, Corey GR.. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis 2014; 58: 10–9. [DOI] [PubMed] [Google Scholar]
- 2. Arias CA, Reyes J, Carvajal LP. et al. A prospective cohort multicenter study of molecular epidemiology and phylogenomics of Staphylococcus aureus bacteremia in nine Latin American countries. Antimicrob Agents Chemother 2017; 61: e00816–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGuinness WA, Malachowa N, DeLeo FR.. Vancomycin resistance in Staphylococcus aureus. Yale J Biol Med 2017; 90: 269–81. [PMC free article] [PubMed] [Google Scholar]
- 4. Hiramatsu K, Hanaki H, Ino T. et al. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 1997; 40: 135–6. [DOI] [PubMed] [Google Scholar]
- 5. Hiramatsu K. Dissemination in Japanese hospital strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 1997; 350: 1670–3. [DOI] [PubMed] [Google Scholar]
- 6. Sievert DM, Rudrik JT, Patel JB. et al. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin Infect Dis 2008; 46: 668–74. [DOI] [PubMed] [Google Scholar]
- 7. Rossi F, Diaz L, Wollam A. et al. Transferable vancomycin resistance in a community-associated MRSA lineage. N Engl J Med 2014; 370: 1524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Panesso D, Planet PJ, Diaz L. et al. Methicillin-susceptible, vancomycin-resistant Staphylococcus aureus, Brazil. Emerg Infect Dis 2015; 21: 1844–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Courvalin P. Genetics of glycopeptide resistance in Gram-positive pathogens. Int J Med Microbiol 2005; 294: 479–86. [DOI] [PubMed] [Google Scholar]
- 10. Périchon B, Courvalin P.. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009; 53: 4580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Nineth Edition: M100. 2019.
- 12. Wang Y, Li X, Jiang L. et al. Novel mutation sites in the development of vancomycin- intermediate resistance in Staphylococcus aureus. Front Microbiol 2017; 7: 2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuo M, Cui L, Kim J. et al. Comprehensive identification of mutations responsible for heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA)-to-VISA conversion in laboratory-generated VISA strains derived from hVISA clinical strain Mu3. Antimicrob Agents Chemother 2013; 57: 5843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howden BP, Davies JK, Johnson PDR. et al. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev 2010; 23: 99–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui L, Iwamoto A, Lian J. et al. Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 2006; 50: 428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cafiso V, Bertuccio T, Spina D. et al. Modulating activity of vancomycin and daptomycin on the expression of autolysis cell-wall turnover and membrane charge genes in hVISA and VISA strains. PLoS One 2012; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howden BP, Peleg AY, Stinear TP.. The evolution of vancomycin intermediate Staphylococcus aureus (VISA) and heterogenous-VISA. Infect Genet Evol 2014; 21: 575–82. [DOI] [PubMed] [Google Scholar]
- 18. Kim T, Kim ES, Park SY. et al. Phenotypic changes of methicillin-resistant Staphylococcus aureus during vancomycin therapy for persistent bacteraemia and related clinical outcome. Eur J Clin Microbiol Infect Dis 2017; 36: 1473–81. [DOI] [PubMed] [Google Scholar]
- 19. Yang CC, Sy CL, Huang YC. et al. Risk factors of treatment failure and 30-day mortality in patients with bacteremia due to MRSA with reduced vancomycin susceptibility. Sci Rep 2018; 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Satola SW, Farley MM, Anderson KF. et al. Comparison of detection methods for heteroresistant vancomycin-intermediate Staphylococcus aureus, with the population analysis profile method as the reference method. J Clin Microbiol 2011; 49: 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0. 2019.
- 22. Wootton M. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother 2001; 47: 399–403. [DOI] [PubMed] [Google Scholar]
- 23. Zhang S, Sun X, Chang W. et al. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS One 2015; 10: e0136082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanaki H, Cui L, Ikeda-Dantsuji Y. et al. Antibiotic susceptibility survey of blood-borne MRSA isolates in Japan from 2008 through 2011. J Infect Chemother 2014; 20: 527–34. [DOI] [PubMed] [Google Scholar]
- 25. Campanile F, Bongiorno D, Falcone M. et al. Changing Italian nosocomial-community trends and heteroresistance in Staphylococcus aureus from bacteremia and endocarditis. Eur J Clin Microbiol Infect Dis 2012; 31: 739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di Gregorio S, Famiglietti A, Vay C. et al. Clinical, microbiological, and genetic characteristics of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia in a teaching hospital. Microb Drug Resist 2015; 21: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vega F, Domínguez M, Bello H. et al. Isolation of Staphylococcus aureus hetero-resistant to vancomycin (hVISA) in the Regional Hospital of Concepción, Chile. Rev Chil Infectol 2015; 32: 588–90. [DOI] [PubMed] [Google Scholar]
- 28. Mendes RE, Sader HS, Deshpande LM. et al. Characterization of baseline methicillin-resistant Staphylococcus aureus isolates recovered from phase IV clinical trial for linezolid. J Clin Microbiol 2010; 48: 568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Da Costa TM, Morgado PGM, Cavalcante FS. et al. Clinical and microbiological characteristics of heteroresistant and vancomycin-intermediate Staphylococcus aureus from bloodstream infections in a Brazilian teaching hospital. PLoS One 2016; 11: e0160506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silveira AC, Cunha GR, Caierão J. et al. Molecular epidemiology of heteroresistant vancomycin-intermediate Staphylococcus aureus in Brazil. Brazilian J Infect Dis 2015; 19: 466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reyes J, Rincón S, Díaz L. et al. Dissemination of methicillin‐resistant Staphylococcus aureus USA300 sequence type 8 lineage in Latin America. Clin Infect Dis 2009; 49: 1861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oliveira DC, De Lencastre H.. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2002; 46: 2155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tenover FC, Arbeit RD, Goering RV. et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995; 33: 2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larsen MV, Cosentino S, Rasmussen S. et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 2012; 50: 1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khatib R, Riederer K, Sharma M. et al. Screening for intermediately vancomycin-susceptible and vancomycin-heteroresistant Staphylococcus aureus by use of vancomycin-supplemented brain heart infusion agar biplates: defining growth interpretation criteria based on gold standard confirmation. J Clin Microbiol 2015; 53: 3543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leonard SN, Rossi KL, Newton KL. et al. Evaluation of the Etest GRD for the detection of Staphylococcus aureus with reduced susceptibility to glycopeptides. J Antimicrob Chemother 2009; 63: 489–92. [DOI] [PubMed] [Google Scholar]
- 37. Nigo M, Diaz L, Carvajal LP. et al. Ceftaroline-resistant, daptomycin-tolerant, and heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus causing infective endocarditis. Antimicrob Agents Chemother 2017; 61: e01235–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bolger AM, Lohse M, Usadel B.. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014; 30: 2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bankevich A, Nurk S, Antipov D. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19: 455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milheiriço C, Oliveira DC, de Lencastre H.. Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphylococcus aureus: ‘SCCmec IV multiplex’. J Antimicrob Chemother 2007; 60: 42–8. [DOI] [PubMed] [Google Scholar]
- 41. McClure-Warnier J-A, Conly J, Zhang K.. Multiplex PCR assay for typing of Staphylococcal Cassette Chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J Vis Exp 2013; 79: 50779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zankari E, Hasman H, Cosentino S. et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 2012; 67: 2640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Camacho C, Coulouris G, Avagyan V. et al. BLAST+: architecture and applications. BMC Bioinformatics 2009; 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Overbeek R, Olson R, Pusch GD. et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 2014; 42: 206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Page AJ, Cummins CA, Hunt M. et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015; 31: 3691–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acid Res 2004; 32: 1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30: 1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Letunic I, Bork P.. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 2016; 44: W242–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing, 2013.
- 50. Boyle-Vavra S, Yin S, Jo DS. et al. VraT/YvqF is required for methicillin resistance and activation of the VraSR regulon in Staphylococcus aureus. Antimicrob Agents Chemother 2013; 57: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Katayama Y, Sekine M, Hishinuma T. et al. Complete reconstitution of the vancomycin-intermediate Staphylococcus aureus phenotype of strain Mu50 in vancomycin-susceptible S. aureus. Antimicrob Agents Chemother 2016; 60: 3730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matsuo M, Hishinuma T, Katayama Y. et al. Mutation of RNA polymerase β subunit (rpoB) promotes hVISA-to-VISA phenotypic conversion of strain Mu3. Antimicrob Agents Chemother 2011; 55: 4188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Silveira AC, Sambrano GE, Paim TG. et al. Is prediffusion test an alternative to improve accuracy in screening hVISA strains and to detect susceptibility to glycopeptides/lipopeptides? Diagn Microbiol Infect Dis 2014; 79: 401–4. [DOI] [PubMed] [Google Scholar]
- 54. Adam HJ, Louie L, Watt C. et al. Detection and characterization of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates in Canada: results from the Canadian Nosocomial Infection Surveillance Program, 1995-2006. Antimicrob Agents Chemother 2010; 54: 945–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takada H, Yoshikawa H.. Essentiality and function of WalK/WalR two-component system: the past, present, and future of research. Biosci Biotechnol Biochem 2018; 82: 741–51. [DOI] [PubMed] [Google Scholar]
- 56. Msadek T, Dubrac S.. Tearing down the wall: peptidoglycan metabolism and the WalK/WalR (YycG/YycF) essential two-component system. Adv Exp Med Biol 2008; 631: 214–28. [DOI] [PubMed] [Google Scholar]
- 57. Liu J, Gefen O, Ronin I. et al. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 2020; 367: 200–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.