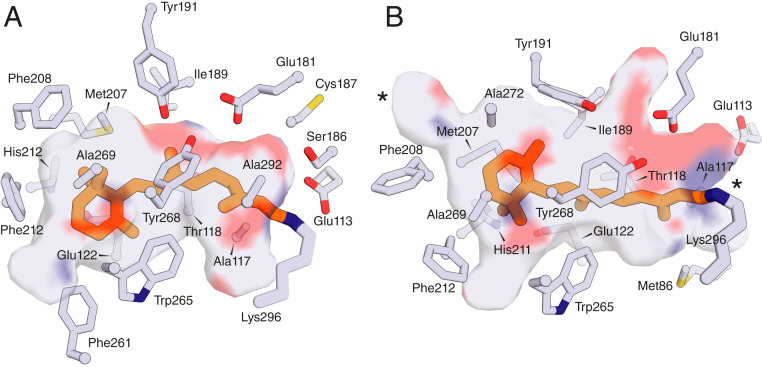
Fig. 2.
Distinct binding pocket geometries for all-trans and 11-cis-retinal as observed in the bovine opsin crystal structure. (A) The crystal structure of ground-state bovine rhodopsin (PDB accession code: 1U19) reveals the 11-cis-retinal chromophore (orange sticks) housed within a bent binding pocket that protects the Schiff base-linked ligand from hydrolysis and spontaneous thermal isomerization. (B) The structure of meta-II rhodopsin (PDB accession code: 3PXD) reveals a more extended binding pocket that accommodates the transform of retinal (orange stick). The pocket rearrangement results in openings to the protein exterior (locations marked by asterisks) that likely enable hydrolysis and release of retinal so that it can be reisomerized to the 11-cis configuration.