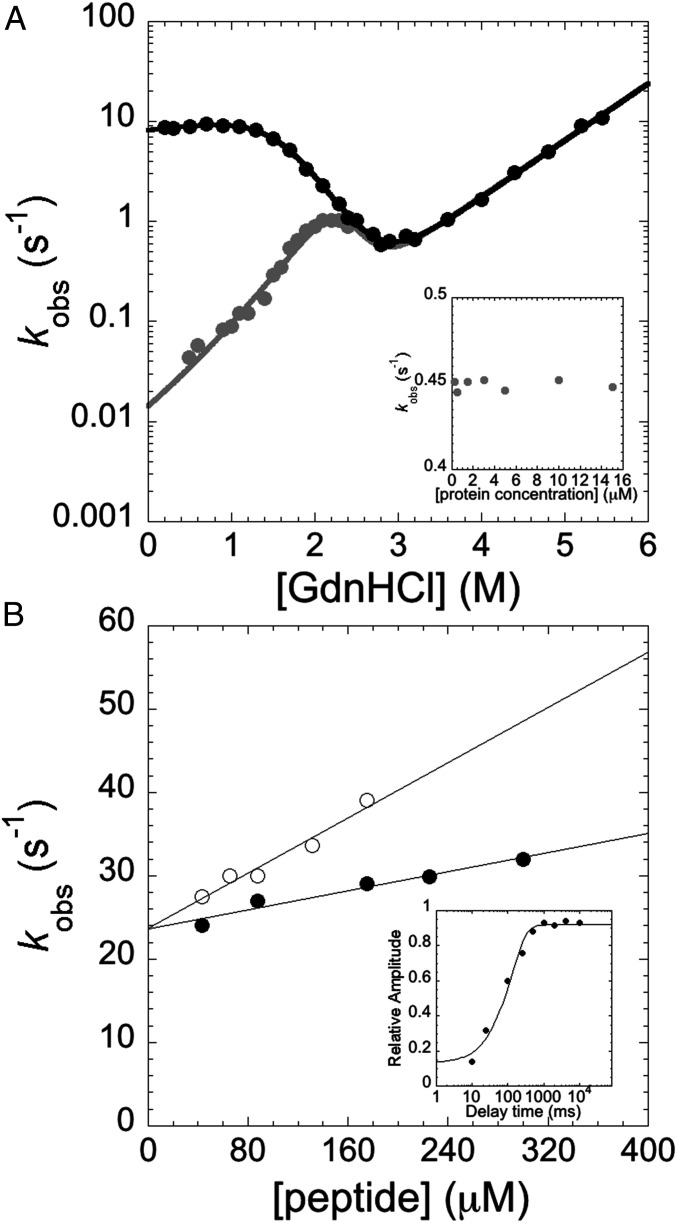
Fig. 4.
(A) Unfolding and refolding kinetics of pP1-P2 in the presence of a stabilizing agent (0.5 M Na2SO4). pP1-P2 was denatured in a moderate (2.2 M; black circles) or high (5.37 M; gray circles) GdnHCl concentration. The refolding of pP1-P2 in the latter condition was slowed by more than two orders of magnitude by the presence of Na2SO4. Data were fitted as described in Materials and Methods. (Inset) Refolding kinetics of pP1-P2 measured at 0.5 M GdnHCl, starting from high denaturant concentrations, at protein concentrations ranging from 250 nM to 15 μM. As expected from a monomolecular folding reaction, rate constants were found to be insensitive to protein concentration. (B) Binding experiments measured by FRET between the Trp (donor) and dansyl (acceptor) attached to the Sans peptide. Empty circles represent the pseudo-first-order plot of the binding experiment between pP1-P2 and the peptide, performed at 25 °C in buffer composed of 50 mM Tris⋅HCl, 0.5 M Na2SO4, and 0.54 M GdnHCl. The binding between the peptide and the misfolded intermediate of pP1-P2 was obtained by a double-jump stopped-flow experiment (black circles). (Inset) Dependence of the relative amplitude of native-like binding competent species as a function of delay time between the first (refolding) and second (binding) mixes. In this case, pP1-P2 denatured in 2.2 M GdnHCl was mixed with refolding buffer and then challenged with 200 μM peptide.