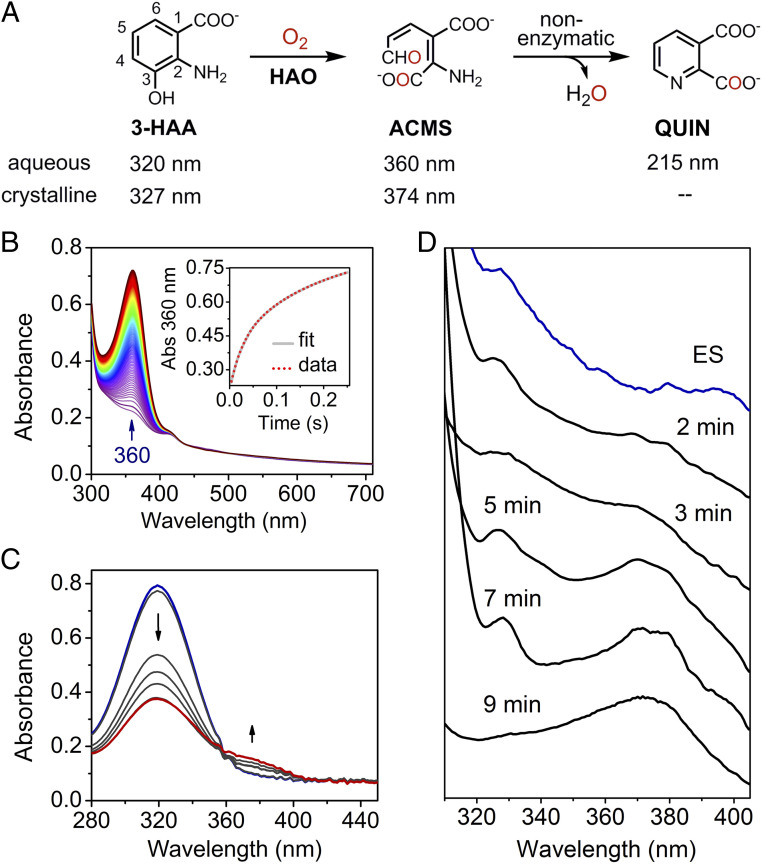
Fig. 1.
Crystalline state of HAO alters the reaction rate and populates catalytic intermediates that are kinetically unresolved in the solution state. (A) The chemical reaction catalyzed by HAO and the nonenzymatic cyclization. (B) Reaction of anaerobically premixed enzyme–substrate complex (0.10 mM) and O2 (5 mM, generated by chlorite dismutase) in solution monitored by stopped-flow photodiode array spectroscopy. The Inset shows the time course of ACMS production monitored by the single-wavelength detector at 360 nm, fitted with the double-exponential function. (C) The catalytic activity of the HAO single crystals was monitored at room temperature by a benchtop spectrophotometer. Seven crystals were incubated in crystallization mother liquor (pH 8.5) with the cryoprotectant described in Experimental Procedures, 3-HAA (0.3 mM), and O2 (1.3 mM). (D) Representative absorption spectra of time-resolved in crystallo reaction obtained from single-crystal UV-vis microspectroscopy. Each spectrum was collected from one single crystal at 100 K.