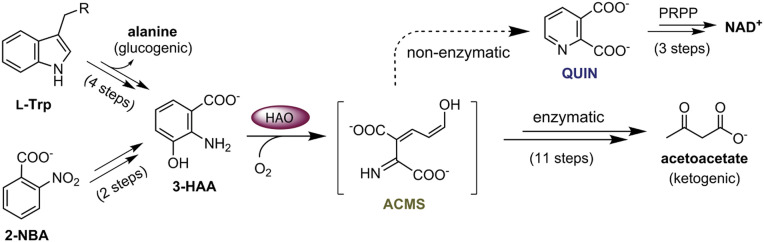
Scheme 1.
The l-tryptophan kynurenine pathway and the bacterial 2-nitrobenzoic acid (2-NBA) biodegradation pathway converge at the aromatic metabolite 3-hydroxyanthranilic acid (3-HAA). The nonheme iron-dependent enzyme, 3-hydroxyanthranilic acid 3,4-dioxygenase (HAO), sits at the critical junction that directs most of the metabolic flux to the enzyme-controlled ketogenic pathway, which branches off for de novo biosynthesis of NAD+ via quinolinic acid (QUIN). The precise conformation of the ring-cleaved product of the HAO reaction, 2-amino-3-carboxymuconic semialdehyde (ACMS), is critical for determining the partitioning of the metabolite flux at the pathway junction for enzymatic and nonenzymatic routes.