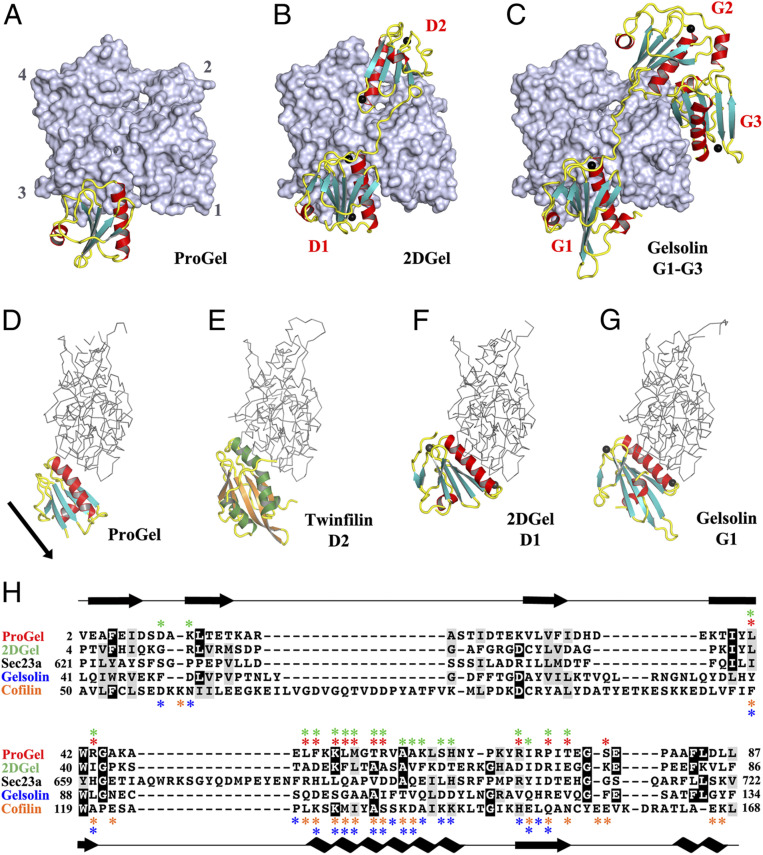
Fig. 4.
The structures of ProGel and 2DGel in complex with rActin. (A) The ProGel/rActin complex. rActin is shown as a surface and ProGel is in schematic representation. (B) The structure of the 2DGel/rActin complex. The four calcium ions associated with 2DGel are shown as black spheres. The crystal structure of 2DGel3/rActin complex is found in SI Appendix, Fig. S7A. (C) The structure of the first three domains of human gelsolin in complex with rActin for comparison (PDB ID code 1EQY). (D–G) Side views of (D) ProGel, (E) twinfilin domain 2 (D2), a cofilin-family member (PDB ID code 3DAW), (F) 2DGel domain 1 (D1), and (G) gelsolin domain G1, in complex with actin. Actin is shown as a trace. Similar representations for 2DGel3 is found in SI Appendix, Fig. S7B. The arrow indicates the displacement of ProGel relative to G1. Data collection and refinement statistics are found in SI Appendix, Table S1. (H) Structure-based sequence alignment of the core region of the gelsolin/cofilin domain of ProGel, 2DGel, Sec23a (which has not been shown to bind actin), hGelsolin, and cofilin. rActin interacting residues are indicated by stars. Asgard proteins above the alignment ProGel, red; 2DGel, green) and human proteins below (gelsolin, blue; cofilin, orange). The consensus secondary structure is shown in black.