Abstract
Mesenchymal stem cell (MSC) research has demonstrated the potential of these cells to modulate lung inflammatory processes and tissue repair; however, the underlying mechanisms and treatment durability remain unknown. Here, we investigated the therapeutic potential of human bone marrow-derived MSCs in the inflammatory process and pulmonary remodeling of asthmatic BALB/c mice up to 14 d after transplantation. Our study used ovalbumin to induce allergic asthma in male BALB/c mice. MSCs were injected intratracheally in the asthma groups. Bronchoalveolar lavage fluid (BALF) was collected, and cytology was performed to measure the total protein, hydrogen peroxide (H2O2), and proinflammatory (IL-5, IL-13, and IL-17A) and anti-inflammatory (IL-10) interleukin (IL) levels. The lungs were removed for the histopathological evaluation. On day zero, the eosinophil and lymphochte percentages, total protein concentrations, and IL-13 and IL-17A levels in the BALF were significantly increased in the asthma group, proving the efficacy of the experimental model of allergic asthma. On day 7, the MSC-treated group exhibited significant reductions in the eosinophil, lymphocyte, total protein, H2O2, IL-5, IL-13, and IL-17A levels in the BALF, while the IL-10 levels were significantly increased. On day 14, the total cell numbers and lymphocyte, total protein, IL-13, and IL-17A levels in the BALF in the MSC-treated group were significantly decreased. A significant decrease in airway remodeling was observed on days 7 and 14 in almost all bronchioles, which showed reduced inflammatory infiltration, collagen deposition, muscle and epithelial thickening, and mucus production. These results demonstrate that treatment with a single injection of MSCs reduces the pathophysiological events occurring in an experimental model of allergic asthma by controlling the inflammatory process up to 14 d after transplantation.
Keywords: asthma, inflammation, lung remodeling, mesenchymal stem cells, immunomodulation
Introduction
Asthma is a chronic inflammatory disease of the airways characterized by recurrent episodes of airflow obstruction and reversible respiratory difficulties1. Asthma affects more than 300 million people worldwide2, and this number is estimated to increase to 400 million by 2025 because of urban development3.
Airway inflammation plays a central role in the pathophysiology of asthma and mainly occurs through the TH2 immune response4. Perpetuation of the inflammatory process promotes significant structural alterations in the lung architecture that are induced by repeated lesions and repair5.
Although the drugs available for asthma treatment (corticosteroids and bronchodilators) promote symptom relief and control inflammation, these drugs do not reverse the changes resulting from airway remodeling. Moreover, prolonged use of corticosteroids can cause adverse effects and result in patients becoming refractory to treatment6,7.
New therapeutic modalities for lung diseases, such as cell therapy, have been experimentally and clinically tested8. Mesenchymal stem cells (MSCs) have attracted great interest in regenerative medicine due to their biological properties, immunomodulatory activities9, and low immunogenicity in autologous, allogeneic, and xenogeneic transplants10. In addition, MSCs secrete several bioactive factors that suppress the local immune system and interfere with important processes involved in tissue repair11. Such effects have already been demonstrated in models investigating MSCs in asthma. After transplantation, MSCs reduced the number of inflammatory cells in the lungs and the levels of proinflammatory biomarkers while alleviating histopathologic modifications12,13. Abreu et al.14 demonstrated some effects of murine MSCs 10 d after transplantation, but whether the effects of MSCs on lung inflammation persist after the study periods previously analyzed has not been demonstrated.
Thus, the objective of the present study was to investigate the therapeutic potential of human bone marrow-derived MSCs in the inflammatory process and pulmonary remodeling in asthmatic BALB/c mice up to 14 d after the MSC transplantation. We hypothesized that the effects of MSCs can minimize changes resulting from pulmonary remodeling, thus bypassing the inflammatory process 7 and 14 d after transplantation.
Materials and Methods
This study was approved by the Ethics Committee in Human Research (approval number 04425212.6.0000.0020) and the Ethics Committee on the Use of Animals (approval number 724) of the Pontifícia Universidade Católica do Paraná (PUCPR) for the use of MSCs from human bone marrow and all animal experimental procedures, respectively.
Obtaining MSCs from Human Bone Marrow
A 5-ml volume of bone marrow was obtained from four human patients. Mononuclear cells were isolated using a Histopaque density gradient (Sigma-Aldrich, São Paulo, SP, Brazil) of 1,077 g/ml. MSC culture was performed as described by Rebelatto et al.15. The remaining MSCs were cultured until the number of cells was sufficient for transplantation, which occurred between passages 3 and 5. At that time, cell characterization and viability assays were performed by flow cytometry.
Characterization of MSCs
On the day of transplantation, MSCs were characterized according to the markers defined by the International Society for Cell Therapy (ISCT)16. The cells were incubated with the following monoclonal antibodies: anti-CD14, anti-CD19, and anti-CD45 antibodies conjugated to fluorescein isothiocyanate (FITC); anti-CD73, anti-CD90, and anti-CD166 antibodies conjugated to phycoerythrin (PE); anti-HLA-DR conjugated to phycoerythrin-cyanine 5 (PE-Cy5); and anti-CD29, anti-CD34, and anti-CD105 conjugated to allophycocyanin (APC). Additionally, cell viability was assessed by Annexin-V and the nucleic acid dye 7-amino actinomycin (7-AAD). Isotype controls for each fluorochrome were used in the reactions. All reagents were produced by BD Pharmingen (BD Biosciences, San Diego, CA, USA). Samples were acquired using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo software v8.0.2 (Tree Star, Ashland, OR, USA).
Experimental Design
The study included 64 six- to eight-wk-old male BALB/c mice weighing 30.9 ± 0.98 g from the PUCPR vivarium. The animals were maintained in a controlled environment under a light/dark cycle of 12/12 h, and CR1 Nuvilab feed (Quimtia, Colombo, PR, Brazil) and water were provided ad libitum.
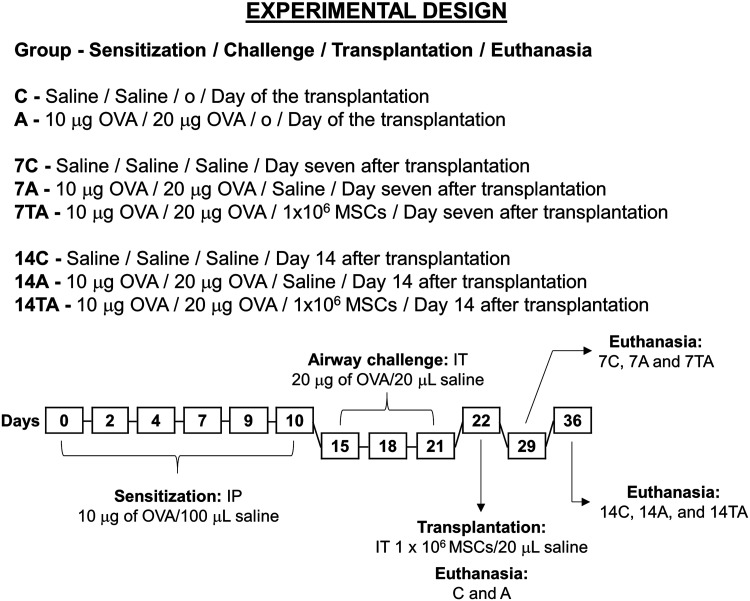
The animals were divided into eight groups (n = 8 per group), which were evaluated at three times. On day zero, i.e., the day of the MSC injection, the control and asthmatic groups (C and A, respectively) were investigated to ensure the efficacy of the protocol. On day 7 after the MSC injection, the control, asthmatic, and treated asthmatic groups were evaluated (7C, 7A, and 7TA, respectively). Three other groups were investigated on day 14 after MSC injection (14C, 14A, and 14TA) (Fig. 1).
Figure 1.
Study design of the experimental model of allergic asthma and posttreatment evaluation of human MSCs in BALB/c mice. Sensitization (days 0 to 10), airway challenge (days 15 to 21), transplantation (day 22), and euthanasia (days 22, 29, and 36)17. The animals were evaluated on day zero: control (C) and asthmatic (A); day 7: control (7C), asthmatic (7A), and treated asthmatic (7TA); and day 14 after transplantation: control (14C), asthmatic (14A), and treated asthmatic groups (14TA). For intratracheal injection, the animals were anesthetized with 100 mg/kg IP ketamine and 10 mg/kg IP xylazine. The procedure was performed through a minor surgery to access the trachea and perform the injection.
IP: intraperitoneal; IT: intratracheal; MSC: mesenchymal stem cell; OVA: ovalbumin.
The experimental model of allergic asthma according to our previous study17 is shown in Fig. 1, which used ovalbumin (OVA) (Sigma-Aldrich, São Paulo, SP, Brazil) for induction. All interventions were performed by the same veterinarian to ensure standardization of the methods used. Briefly, the animals in the A, 7A, 7TA, 14A, and 14TA groups were sensitized with 10 µg of OVA in 100 µl of saline via intraperitoneal (IP) injection on days 0, 2, 4, 7, 9, and 10. The airways of these animals were challenged with 20 µg of OVA in 20 µl of saline administered via intratracheal (IT) injection on days 15, 18, and 21. In these procedures, the C, 7C, and 14C groups received only saline solution. On day 22, the animals in the C and A groups were euthanized, while the 7C, 7A, 14C, and 14A groups received saline via IT injection, and the animals in the 7TA and 14TA groups were transplanted with 1 × 106 MSCs in 20 µl of solution by the same route. On day 29 (7 d after injection), the 7C, 7A, and 7TA group animals were euthanized. The animals in the 14C, 14A, and 14TA groups were euthanized on day 36 (14 d after injection).
Surgical Procedures
The IT OVA administration for the allergen challenge, MSC injection, and blood and bronchoalveolar lavage fluid (BALF) collection were performed under anesthesia with 100 mg/kg 5% ketamine hydrochloride (König, Santana de Parnaíba, SP, Brazil) and 10 mg/kg 2% xylazine hydrochloride (Vetbrands, Paulínia, SP, Brazil). To avoid a reduction in body temperature during anesthesia, the animals were placed in a supine position on a heated table (Master Digital SA-300; Ch@mpion Eletronics, Porto Alegre, RS, Brazil). A small incision (approximately 1 cm) in the skin was performed over the medial ventral cervical region (over the trachea) in the craniocaudal direction. The ventral cervical muscles were divulsed, and after the identification of the trachea, the solutions (Allergenic challenge: saline or OVA; Transplantation: saline or MSCs) were administered by a 0.3-ml ultrafine insulin syringe (Becton Dickinson, Curitiba, PR, Brazil).
Sample Collection and Processing
The animals were anesthetized individually with an overdose of anesthetics in a painless manner, and blood was obtained by intracardiac puncture. Then, BALF was obtained by exposing the trachea, inserting an 18G intravenous catheter (Solidor, Osasco, SP, Brazil), and slowly infusing 1 ml of sterile saline through the catheter and aspirating three times. The recovered BALF was processed as described by Muehlmann et al.18. BALF supernatants were stored at −20°C for total protein, hydrogen peroxide (H2O2), and interleukin (IL) determination. The cell pellet was resuspended in phosphate-buffered saline (Gibco Invitrogen, Carlsbad, CA, USA) for total and differential cytological evaluation.
BALF Total and Cytological Evaluation
BALF total cell counts were performed in a Neubauer chamber (Boeco Germany, Hamburg, HH, Germany), and the results are presented as the number of cells × 104/ml of BALF.
For the lymphocyte and eosinophil evaluation, slides were prepared with 10 µl of the cell pellet and stained by the Romanowski technique (Newprov, Pinhais, PR, Brazil); in total, 200 cells were counted at 1,000× magnification under an Olympus CX41 optical microscope (Olympus, São Paulo, SP, Brazil), and the eosinophils and lymphocytes were determined.
Total Protein Concentration in BALF
The total protein concentration in BALF was determined using the Bradford method19 with Coomassie Brilliant Blue G-250 (Biotec, Pinhais, PR, Brazil). Fluorescence emission was detected with a 595-nm filter in a microplate reader (Nova Analítica, São Paulo, SP, Brazil), and the results are presented in µg/ml.
H2O2 Concentration in BALF
The presence of H2O2 in BALF was evaluated with the Amplex Red Kit (Invitrogen, Eugene, OR, USA) as recommended by the manufacturer. Fluorescence emission was detected with a 580-nm filter in a microplate reader (Nova Analítica, São Paulo, SP, Brazil), and the results are presented in µM.
Quantification of ILs in BALF
The concentrations of pro- (IL-5, IL-13, and IL-17A) and anti-inflammatory (IL-10) ILs in BALF were determined by a CBA Flex Set (BD Biosciences, San Diego, CA, USA) according to the manufacturer’s recommendations. ILs were quantified simultaneously on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo v8.0.2 software (Tree Star, Ashland, OR, USA). The results are presented in pg/ml.
Lung Collection and Histopathological Evaluation
After biological material collection, the mice were humanely euthanized individually in a painless manner. The procedure was performed by anesthetic overdose with 5% ketamine hydrochloride (400 mg/kg/IP) (König, Santana de Parnaíba, SP, Brazil) and 2% xylazine hydrochloride (50 mg/kg/IP) (Vetbrands, Paulínia, SP, Brazil).
The lungs were removed from the thoracic cavity, washed in physiological solution to remove blood from the surface, and fixed in 10% buffered formalin (Biotec, Pinhais, PR, Brazil) for at least 48 h. Then, the whole lungs were transferred to cassettes and processed by histological techniques. Airway remodeling was assessed by sections stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and Masson trichrome (MT). H&E staining was used to evaluate pulmonary morphology, the presence and intensity of inflammatory infiltration in the peribronchiolar region and adjacent area, thickening of the smooth muscle, and changes in the lining epithelium, with an emphasis on cellular desquamation of the bronchiolar lumen. PAS staining was used to investigate mucus and mucus-producing cells (goblet cells), and MT staining revealed collagen deposition in the peribronchiolar region.
Images of 30 bronchioles (15 from each lung) randomly selected from the histological sections were captured by a DP25 digital camera (Olympus, São Paulo, SP, Brazil) coupled to a CX41 optical microscope (Olympus, São Paulo, SP, Brazil). The images were acquired by DP2-BSW software (Olympus, São Paulo, SP, Brazil) at two magnifications: 200× and 400×. Two investigators evaluated the presence and intensity of lung inflammation through semi-quantitative analysis using a scoring system (absent: grade 0, mild: grade 1, moderate: grade 2, and intense: grade 3)17.
Mucus production was estimated by the presence of cells positive for PAS staining in the lung sections and the overlap of a circle divided into eight regions with congruent central angles; this evaluation was adapted from Firinci et al20. The sum of the positively marked areas was categorized according to the scoring system described earlier.
Statistical Analysis
The D’Agostino and Pearson test was applied to evaluate the normality of the distribution of quantitative variables. For parametric data (total cells, differential cytology, proteins, H2O2, and ILs), Student’s t-test was used to compare the means of groups C vs. A and 7TA vs. 14TA, and one-way ANOVA followed by Tukey’s test was used to compare groups 7C vs. 7A vs. 7TA and groups 14C vs. 14A vs. 14TA. The values are expressed as the mean ± standard error of the mean (SEM).
The Mann–Whitney test was used for the histopathological analysis of groups C vs. A and 7TA vs. 14TA, and the Kruskal–Wallis test followed by Dunn’s test for multiple post hoc comparisons was employed to compare groups 7C vs. 7A vs. 7TA and groups 14C vs. 14A vs. 14TA. The results are expressed as the mean ± SEM. Statistical analyses were performed using the software GraphPad Prism version 5.03 for Windows (GraphPad, La Jolla, CA, USA). The results were considered significant when P < 0.05.
Results
Expansion, Immunophenotypic Characterization, and Viability of MSCs
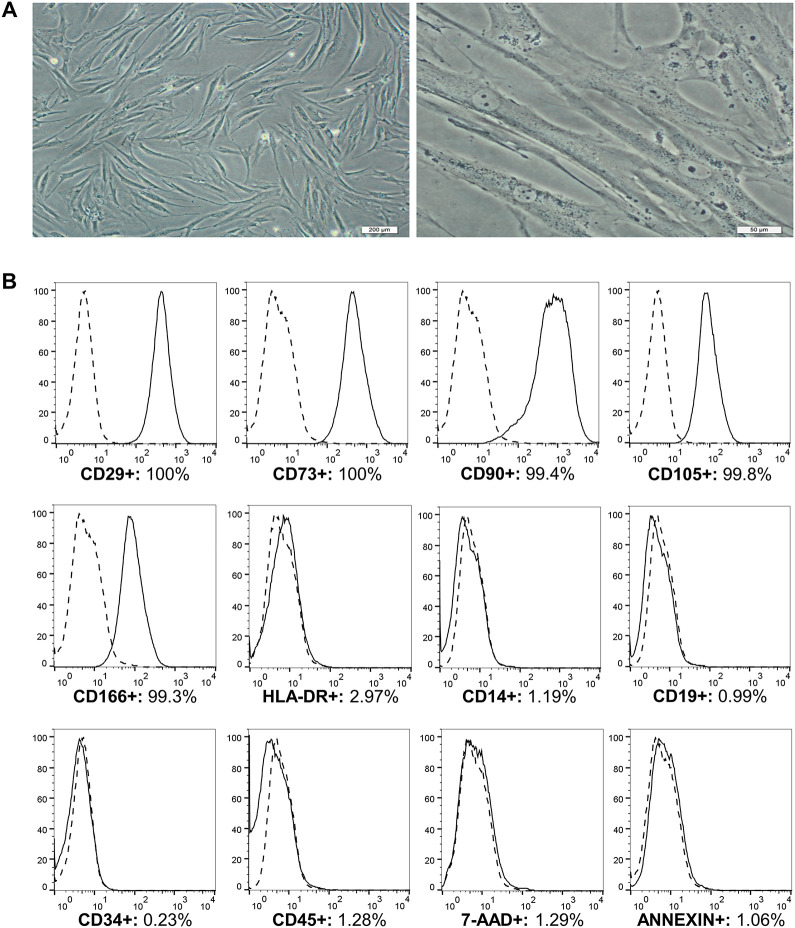
MSCs presented a fibroblast-like morphology when adhered to culture flasks (Fig. 2A). Immunophenotypic characterization of the MSCs was performed in accordance with the ISCT and showed high expression levels of CD29 (99.8%), CD73 (99.8%), CD90 (99.2%), CD105 (99.7%), and CD166 (99.5%) and low expression levels of HLA-DR (2.21%), CD14 (0.85%), CD19 (0.64%), CD34 (0.99%), and CD45 (1.06%). In the third passage, 95.67% of the cell samples were viable, and 0.92% were Annexin-V positive (Fig. 2B).
Figure 2.
MSCs from human bone marrow cultured until passage 3. A. Culture with 90% confluence. The cells adhere to plastic and exhibit a fibroblast-like morphology. Magnification: 100× (left) and 400× (right). B. Markers of MSCs and cell viability from a representative sample of bone marrow. Data shown in the histograms: the isotypic control is presented by a dashed line; the CD29, CD73, CD90, CD105, CD166, HLA-DR, CD14, CD19, CD34, and CD45 markers and the cell viability markers Annexin-V and 7-AAD are presented by a continuous line. The results are expressed as percentages (%).
MSCs: mesenchymal stem cells; 7-AAD: 7-amino actinomycin.
BALF Total and Cytological Findings
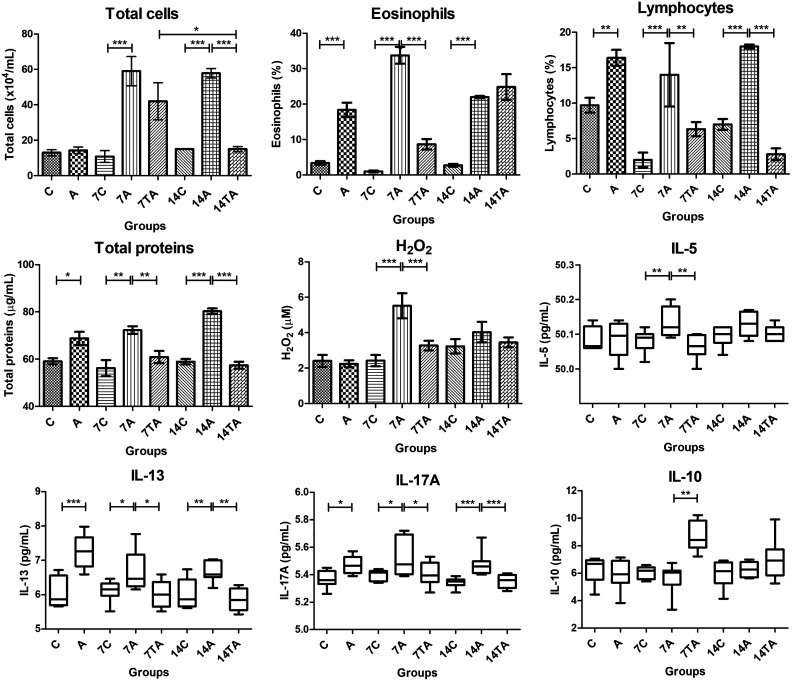
Inflammatory cells were evaluated in the BALF of the animals. On day zero, large numbers of eosinophils (P < 0.0001) and lymphocytes (P = 0.004) were observed in the asthma group (Fig. 3).
Figure 3.
Evaluations of bronchoalveolar lavage fluid: total cells, eosinophils, lymphocytes, total proteins, H2O2, and proinflammatory (IL-5, IL-13, and IL-17A) and anti-inflammatory (IL-10) interleukins. The animals were evaluated on day zero: control (C) and asthmatic (A); day 7: Control (7C), asthmatic (7A), and treated asthmatic (7TA); and day 14 after transplantation: control (14C), asthmatic (14A), and treated asthmatic groups (14TA). Significant results were found in both groups that received mesenchymal stem cell injection compared to the asthma groups. The results are expressed as the mean ± standard error of the mean. *P < 0.05; **P < 0.005; ***P < 0.001.
IL: interleukin.
On day 7, the percentages of total cells (P = 0.0002), eosinophils (P < 0.0001), and lymphocytes (P < 0.0001) in the asthma group were higher than those in the control group (Fig. 3). At the same time point, the treated asthma group exhibited a significantly reduced number of eosinophils (P < 0.0001) and lymphocytes (P = 0.0023) (Fig. 3).
On day 14, high percentages of total cells (P < 0.0001), eosinophils (P < 0.0001), and lymphocytes (P < 0.0001) were observed in the airways in the asthma group (Fig. 3). The animals in the treated group exhibited significantly reduced numbers of total cells (P < 0.0001) and lymphocytes (P < 0.0001) in the BALF (Fig. 3).
A significant difference in total cells was found in the treated asthma groups. The animals in the treated groups showed lower cell counts at 14 d than those at 7 d (P = 0.012) (Fig. 3).
Total Protein and H2O2 Concentrations in BALF
The BALF total protein concentration was increased in the asthma groups at days zero (P = 0.0058), 7 (P = 0.0011), and 14 (P < 0.0001). The MSC-treated groups exhibited significantly reduced protein concentrations at days 7 (P = 0.0025) and 14 (P < 0.0001) (Fig. 3).
We did not observe a difference in H2O2 concentrations in the groups on day zero and at 14 d. Nevertheless, on day 7, higher levels of hydrogen peroxide were detected in the asthma group (P = 0.0005) than in the control group, with significantly lower values in the animals that received MSC transplantation (P = 0.0005) (Fig. 3).
IL Quantification in BALF
Proinflammatory cytokine analysis of BALF samples showed high levels of IL-13 (P = 0.0003) and IL-17A (P = 0.0055) in the asthma group on day zero (Fig. 3).
On day 7, compared to the control group, elevated levels of IL-5 (P = 0.0023), IL-13 (P = 0.0134), and IL-17A (P = 0.0243) were observed in the asthma group, and compared to the untreated group, the MSC transplantation in the treated asthma group resulted in significant reductions in the IL-5 (P = 0.0023), IL-13 (P = 0.0134), and IL-17A (P = 0.0243) levels (Fig. 3).
Asthma group animals evaluated on day 14 exhibited higher levels of IL-13 (P = 0.0012) and IL-17A (P = 0.0003) than the control group, and the treated group demonstrated lower levels of both IL-13 (P = 0.0012) and IL-17A (P = 0.0003) in BALF than did the untreated group (Fig. 3).
A significant difference in IL-5 levels was found in the treated asthma groups. The animals showed higher results at 14 d than those at 7 d (P = 0.0362) (Fig. 3).
Interestingly, on day 7, significant increases in the anti-inflammatory cytokine IL-10 were observed in the BALF of animals treated with MSCs (P < 0.0001) (Fig. 3).
Airway Remodeling
The lung histopathological findings of the studied specimens showed features of lung remodeling in the animals with induced asthma at the three evaluated times and decreases in most of these alterations in the mice treated with MSCs (Figs. 4 and 5).
Figure 4.
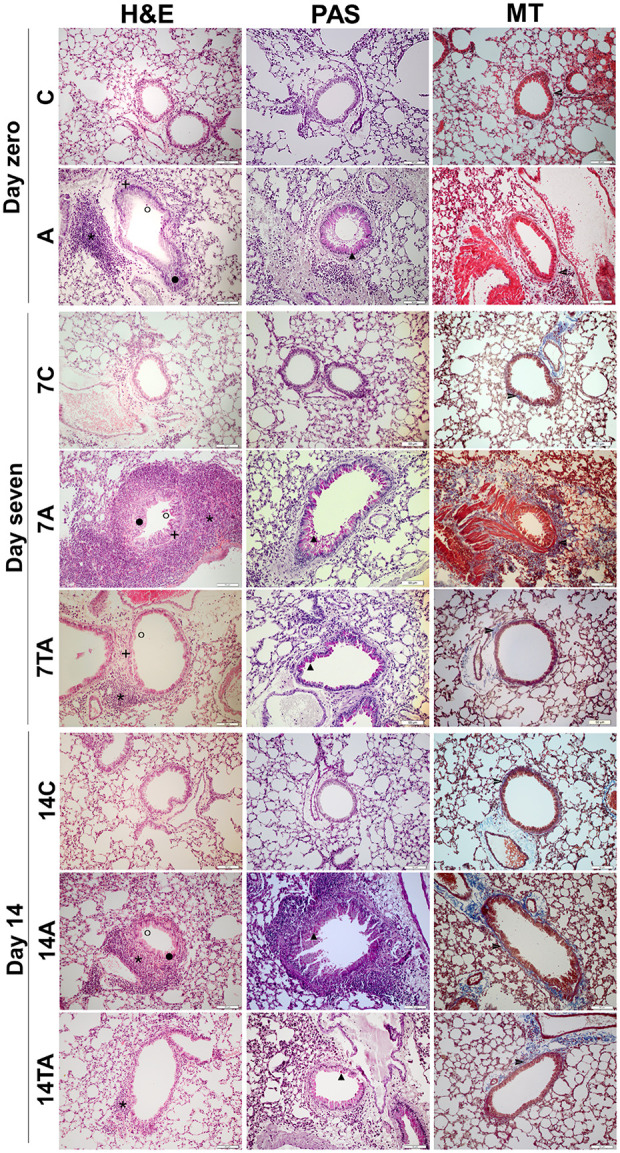
Histopathological evaluation of lung sections. 1. H&E. 2. PAS. 3. MT.
The animals were evaluated on day zero: control (C) and asthmatic (A);
day 7: control (7C), asthmatic (7A), and treated asthmatic (7TA); and
day 14 after transplantation: control (14C), asthmatic (14A), and
treated asthmatic groups (14TA). The control groups exhibited preserved
lungs, and the animals in the asthma groups showed alterations involving
lung remodeling on days 7 and 14: * Inflammatory infiltration, + muscle
thickening,  epithelial
thickening, ˆ epithelial desquamation, ▴ mucus production, and
epithelial
thickening, ˆ epithelial desquamation, ▴ mucus production, and
 collagen deposition. These changes are less evident in the treatment
groups. Magnification: 200×.
collagen deposition. These changes are less evident in the treatment
groups. Magnification: 200×.
H&E: Hematoxylin and eosin; MT: Masson trichrome; PAS: Periodic acid-Schiff.
Figure 5.
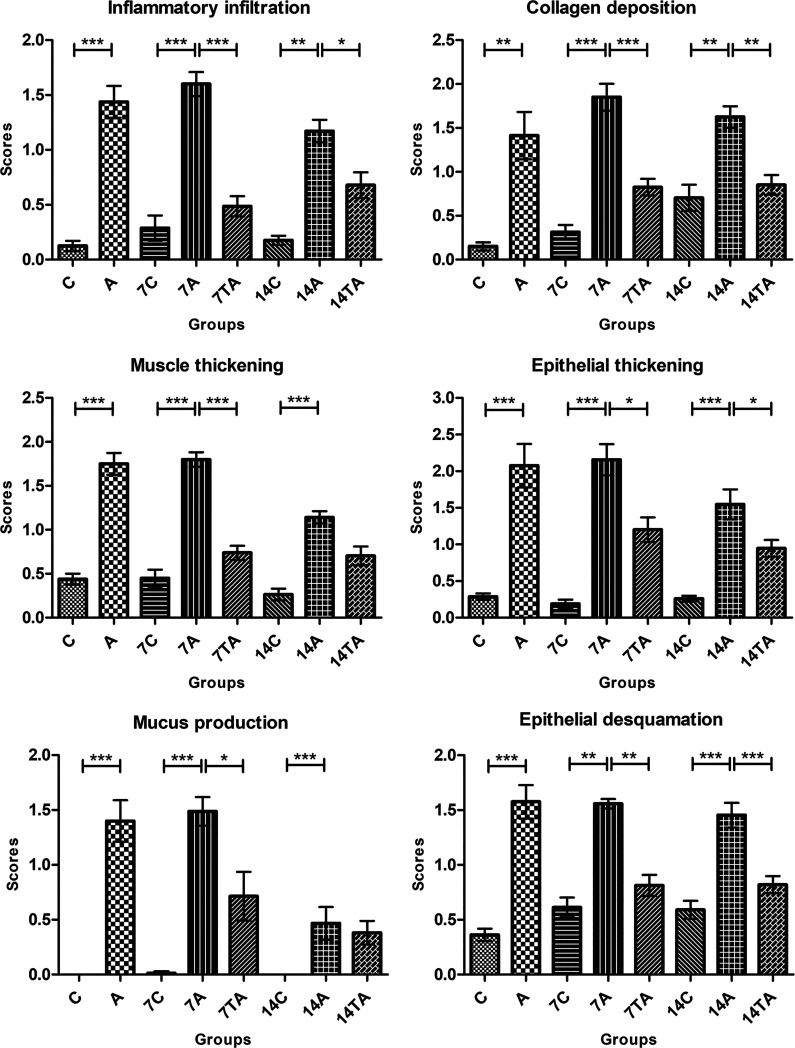
Histopathological analyses of lung remodeling. The animals were evaluated on day zero: control (C) and asthmatic (A); day 7: control (7C), asthmatic (7A), and treated asthmatic (7TA); and day 14 after transplantation: control (14C), asthmatic (14A), and treated asthmatic groups (14TA). The treatment led to statistically significant changes in almost all evaluated parameters. The results are expressed as the mean ± standard error of the mean. *P < 0.05; **P < 0.005; ***P < 0.001.
On day zero, the asthma group showed significant changes in inflammatory infiltration (P = 0.0009), collagen deposition (P = 0.0011), muscle thickening (P = 0.0009), epithelial thickening (P = 0.0009), mucus production (P = 0.0004), and epithelial desquamation (P = 0.0009).
On day 7, the animals in the asthma group continued to exhibit intense inflammatory infiltration (P = 0.0002), collagen deposition (P = 0.0002), muscle layer thickening (P = 0.0002), epithelial thickening (P = 0.0001), mucus production by goblet cells (P = 0.0004), and epithelial desquamation (P = 0.0014). However, the group treated with MSCs demonstrated significantly reduced lung remodeling compared to that in the untreated animals in terms of inflammatory cells (P = 0.0002), collagen deposition (P = 0.0002), muscle (P = 0.0002) and epithelial (P = 0.031) thickening, mucus production (P = 0.0237), and epithelial desquamation (P = 0.0014).
At day 14, airway remodeling remained very strong in the asthma group in terms of all evaluated criteria: inflammatory infiltration (P = 0.0044), collagen deposition (P = 0.0042), muscle (P = 0.0002) and epithelial (P = 0.0003) thickening, mucus production by goblet cells (P = 0.0008), and epithelial desquamation (P = 0.0004). However, except for muscle thickening and mucus production, significant decreases were verified in the treated group for the modifications of the lung tissue: inflammatory infiltration (P = 0.0235), collagen deposition (P = 0.0042), epithelial thickening (P = 0.0353), and epithelial desquamation (P = 0.0004).
Discussion
In the present study, we developed an experimental model of allergic asthma and investigated important factors in the inflammatory process responsible for inducing disease. However, our focus was pulmonary remodeling. We evaluated several histopathological aspects to answer two questions: Would the immune systems of the animals challenged with OVA but without treatment fight the allergen? Would MSC treatment of the animals with induced asthma minimize the changes resulting from pulmonary remodeling, thus bypassing the inflammatory process? To answer these questions, we evaluated some factors that influence pulmonary remodeling through inflammatory responses, mainly in BALF.
Therefore, our study used BALB/c mice to generate an asthma animal model with OVA as the allergen, which is considered the gold standard for this type of investigation21. Changes in the clinical characteristics of asthma are scarcely observed in these animals, which may complicate the comparison of the results to the clinical manifestations observed in patients with asthma22. The animals were repeatedly exposed to OVA during the sensitization stages and exhibited diffuse pulmonary inflammatory processes consistent with asthma, reflecting the reliability of the treatment results21,22.
Moreover, IT airway allergenic challenges have also been applied in other studies14,23 and they simulate the exacerbation of asthma that occurs when people inhale an allergen24. IT MSC transplantation has also been used successfully in similar studies as this route delivers cells directly to the affected airways25,26. Intravenous MSC administration is also an option in protocols such as ours27. However, intravenous MSC administration causes most cells to be trapped in the lungs28, which may be advantageous for treating asthma but still requires further investigation for undesirable side effects, such as stroke29 and thromboembolism30. For these reasons, we selected IT injection to treat mice with induced asthma.
In addition, our study used xenogeneic transplantation with human bone marrow-derived MSCs, which is preferred by many researchers who use mouse asthma models. The positive effects of MSCs on the immune systems of other animal species are well demonstrated12,31,32; thus, the protocol used in the present study was a safe option.
Another relevant feature of our study, which we did not find in other previously published studies, is our investigation of the disease at different times: days zero, 7, and 14 after treatment. These evaluations were important because they helped us to understand the extent of the benefits of a single injection of MSCs and to evaluate the therapeutic results because euthanizing some animals at day zero allowed us to verify model establishment, thus providing the foundation for this experiment.
The first modification observed in the asthma groups was the large number of total cells in the BALF at 7 and 14 d. These results corroborate the severe inflammatory infiltration in the lung tissue at the three evaluated times. Furthermore, an increase in the permeability of the alveolar–capillary barrier due to the increased total protein levels was a result of the stimulated inflammatory process33,34.
Additionally, the cellular influx into the lungs of the asthmatic animals was dominated by an increase in lymphocytes and eosinophils, which is characteristic of the TH2 immune response in this model35. The eosinophilic pattern represents an important event in allergic asthma and has been correlated with intense airway inflammation in several studies36–39. This feature was observed in the asthmatic groups until 14 d. The chemotaxis of eosinophils to the lungs is mediated by IL-5, and we observed increases in IL-5 levels at 7 d.
Eosinophil activation in the lung increases the production of reactive oxygen species and their participation in oxidative stress. We investigated hydrogen peroxide, which can originate from hydroxyl radicals that are highly harmful to lung tissue40. The increased levels of H2O2 reveal one of the pathways through which pulmonary damage may occur41, as observed in our histopathological results and in another study42.
In our study, a significant increase in IL-13 was observed in the OVA-challenged animals compared to nonchallenged animals. Tissue damage was also caused by increased IL production, which is highly involved in the asthma inflammatory process43. IL-13 is related to airway hyperresponsiveness44, which was not measured in this study. However, IL-13 is involved in modifications observed in lung remodeling45, such as thickening of the smooth muscle layer, which favors narrowing of the airways and subsequent hyperresponsiveness46. Indeed, the increased levels of IL-13 observed in the asthmatic mice corroborated the high collagen deposition in the peribronchiolar region observed at all evaluated times. This change can also contribute to hyperresponsiveness and to the development of various levels of subepithelial fibrosis13,47. The results of Mohammadian et al.39 demonstrated lower subepithelial fibrosis in sensitized mice treated with MSC allogeneic combined with simvastatine compared to asthma group.
Our study demonstrated changes in airway tissue related to thickening and epithelial desquamation. Damage to the function of the epithelial barrier associated with asthma occurs due to the action of inflammatory substances and allergens, which increase the permeability of the mucosa48,49. In addition, functional abnormalities of airway epithelial cells can also cause allergic inflammation49. Thus, in the study of Yao et al.50, IT transplantation of induced pluripotent stem cell (iPSC)-MSCs promoted inflammation relief in asthmatic mice and protected epithelial cells by mitochondrial transfer from iPSCs-MSCs to epithelial cells with dysfunctional mitochondria.
Another important aspect was caliciform metaplasia, which was observed at all evaluated times. The increased number of goblet cells, which results in mucosal metaplasia, initially occurs to increase mucus production, improving the clearance of dead cells and pathogens from the airways; however, this change results in luminal obstruction, which compromises gas exchange and aggravates clinical signs of respiratory distress in humans43,51. This characteristic of asthma is known to be mediated by IL-4 and IL-1352. In chronic respiratory diseases, mucosal metaplasia persists and results in airway obstruction, which significantly contributes to morbidity and mortality20,45,51.
The participation of IL-17 cells has been widely discussed because these cells can induce the development of asthma by secreting factors such as IL-17A and IL-17F53,54. Therefore, these factors were quantified in our study. The IL-17A level in the BALF of the animals with asthma in the A group (on the day of transplantation) was higher than that in the control group. The presence of IL-17A has been reported in the lung, sputum, BALF, and serum of patients with asthma55,56. We observed increased IL-17A and IL-5 levels 7 d after transplantation, possibly due to a synergistic effect on the recruitment and activation of eosinophils57.
Treatment of the animals significantly interfered with the inflammatory process of asthma at 7 and 14 d, demonstrating the positive effects of MSCs in the experimental model of allergic asthma. Although we observed increased eosinophil levels after the asthma treatment, the anti-inflammatory action of IL-10 appears to have prevailed; thus, the histopathological findings reveal improved lung remodeling, and although the animals treated with MSCs did not show the same response as that on day 7, most aspects improved significantly.
Significant improvement in the changes was observed according to most of the evaluated criteria in the treated animals compared to the animals that did not receive treatment; these improvements were accompanied by lower levels of IL-5, IL-17A, BALF total proteins, H2O2, and lymphocytes. Additionally, MSC treatment resulted in decreased IL-13 levels in the BALF, which may be associated with improvements in the lung tissue via reductions in inflammatory infiltration, collagen deposition, muscle and epithelial thickening, and epithelial desquamation in the lumen of the bronchioles.
However, on day 14 after MSC transplantation, some of the asthma-related changes recurred, suggesting a limitation in the duration of the treatment period. An increase in the permeability of the alveolar–capillary barrier was observed due to the increase in total protein concentration levels. An increased level of IL-5 was also observed, which coincided with the increase in eosinophil counts in the BALF, and the resulting attraction of eosinophils to the lungs of the animals. With the maintenance of reduced levels of IL-13 and IL-17A, the treatment continued to control tissue damage through reductions in inflammatory infiltration, collagen deposition, epithelial thickening, and epithelial desquamation.
The MSC treatment led to the control of the inflammatory process not only through changes in proinflammatory ILs but also through an increase in the production of the anti-inflammatory interleukin IL-10 in the BALF of the treated animals. IL-10 mediates an important mechanism of immune regulation during airway allergy, regulating the survival of TH2 cells and the severity of TH2-mediated allergic airway inflammation58.
Our results suggest that MSCs control inflammation and tissue repair from the 7th to the 14th day after transplantation. Although some parameters evaluated at 14 d did not present significant differences after treatment, the results were very encouraging. Significant improvement in the respiratory architecture was observed, with evident repair in the epithelial region, reduced collagen deposition, and reduction in lung inflammatory infiltration. These results are interesting because they clearly demonstrated the potential of MSCs to participate in tissue repair by controlling the pathophysiological events involved in asthma.
According to the literature, we understand that the immunomodulatory effects observed in the airways of the asthmatic animals were produced by the paracrine activity of MSCs. Many authors believe that the beneficial effect of MSCs is associated with the soluble factors secreted by cells59,60. Different approaches are being evaluated to elucidate the best strategy for cell therapies in the treatment of various diseases, including asthma61. In contrast to Keyhanmanesh et al.62, who treated asthmatic rats with single or repeated dosages of conditioned medium derived from allogeneic MSCs, in our study, the production and secretion of paracrine factors by MSCs occurred at the site of injury. Our research demonstrates the control of the inflammatory process and pulmonary remodeling. The results obtained by Keyhanmanesh et al.62 indicated that only treatment with repeated dosages of conditioned medium significantly reduced histopathological damage in the asthma model. Therefore, it is important to evaluate the different methodologies used and determine the therapeutic potential of cells and their products for future clinical use.
Obviously, the study has some limitations, and the results obtained should be further studied in depth to determine the most effective use of the treatment. With the results obtained in this research, we identified new questions to be addressed in subsequent steps: When exactly do the therapeutic effects begin to decline? When are the therapeutic effects strongest between the 7th and 14th days after injection? Can a second injection during this time prolong the beneficial effects of MSCs?
Our findings can be translated to clinical research. This approach was outlined by the authors due to the need for new therapeutic modalities that interfere with airway remodeling unlike currently available drugs that act on only the inflammatory process. More than one infusion of MSCs may be more effective and increase the durability of therapeutic effects, but this hypothesis must be further studied. However, the results demonstrated that treatment with a single injection of MSCs reduced the pathophysiological events occurring in the experimental model of allergic asthma by controlling the inflammatory process up to 14 d after transplantation.
Acknowledgments
We would like to thank Bruna Schaidt for experimental assistance, Márcia Olandoski for guidance regarding the statistical analysis, our collaborators in the Pathology Experimental Laboratory of PUCPR for processing the histological specimens, and our collaborators in the PUCPR vivarium for caring for the animals used in this study.
Footnotes
Ethical Approval: The use of MSCs from human bone marrow and all animal experimental procedures performed in this study were approved by the Ethics Committee in Human Research (No: 04425212.6.0000.0020) and the Ethics Committee on the Use of Animals (No: 724) of the Pontifícia Universidade Católica do Paraná (PUCPR), respectively.
Statement of Human and Animal Rights: All procedures performed in this study were conducted in accordance with the Ethics Committee in Human Research (No: 04425212.6.0000.0020) and the Ethics Committee on the Use of Animals (No: 724) of the Pontifícia Universidade Católica do Paraná (PUCPR), Brazil.
Statement of Informed Consent: Written informed consent was obtained from the patients who donated bone marrow volume for this study. Their information were anonymized in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Grants from Fundação Araucária [No. 22701], Curitiba, Paraná, Brazil.
ORCID iD: Lidiane Maria Boldrini-Leite  https://orcid.org/0000-0002-9277-5158
https://orcid.org/0000-0002-9277-5158
References
- 1. Vijverberg SJ, Hilvering B, Raaijmakers JA, Lammers JW, Maitland van der Zee AH, Koenderman L. Clinical utility of asthma biomarkers: from bench tobedside. Biologics. 2013;7(1):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Initiative for Asthma. 2017. http://ginasthma.org/2017-pocket-guidefor-asthma-management-and-prevention/ (accessed 18 April 2019).
- 3. To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet LP. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leite LMB, Michelotto PV, Jr, Moura SAB, Brofman PRS, Senegaglia AC. Airway remodeling in the asthma model: is there standardization in this evaluation? Biomed J Sci & Tech Res. 2019;15(1):11146–11149. [Google Scholar]
- 5. Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367(3):551–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, de Jongste JC, Kerstjens HA, et al. A new perspective on concepts of asthma severity andcontrol. Eur Respir J. 2008;32(3):545–554. [DOI] [PubMed] [Google Scholar]
- 7. Alves RSA, Vianna FAF, Pereira CAC. Clinical phenotypes of severe asthma. J Bras Pneumol. 2009;34(9):646–653. [DOI] [PubMed] [Google Scholar]
- 8. Weiss DJ. Concise review: current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells. 2014;32(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54(5):1418–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Huang X, Wang H, Liu X, Zhang T, Wang Y, Hu D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther. 2015;6(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. [DOI] [PubMed] [Google Scholar]
- 12. Chan CK, Lin TC, Huang YA, Chen YS, Wu CL, Lo HY, Kuo ML, Wu KH, Huang JL. The modulation of Th2 immune pathway in the immunosuppressive effect of human umbilical cord mesenchymal stem cells in a murine asthmatic model. Inflamm Res. 2016;65(10):795–801. [DOI] [PubMed] [Google Scholar]
- 13. Kang SY, Park DE, Song WJ, Bae BR, Lee JW, Sohn KH, Lee HS, Kang HR, Park HW, Chang YS, Choi SJ, et al. Immunologic regulatory effects of human umbilical cord blood-derived mesenchymal stem cells in a murine ovalbumin asthma model. Clin Exp Allergy. 2017;47(7):1–9. [DOI] [PubMed] [Google Scholar]
- 14. Abreu SC, Antunes MA, Xisto DG, Cruz FF, Branco VC, Bandeira E, Zola Kitoko J, de Araújo AF, Dellatorre-Texeira L, Olsen PC, Weiss DJ, et al. Bone Marrow, adipose, and lung tissue-derived murine mesenchymal stromal cells release different mediators and differentially affect airway and lung parenchyma in experimental asthma. Stem Cells Transl Med. 2017;6(6):1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rebelatto CK, Aguiar AM, Moretão MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2008;233(7):901–913. [DOI] [PubMed] [Google Scholar]
- 16. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells the international Society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 17. Leite LMB, Michelotto PV, Jr, Moura SAB, Capriglione LGA, Fragoso FYI, Pimpão CT, Senegaglia AC, Brofman PRS. Histopathologic evaluation, anesthetic protocol, and physiological parameters for an experimental Balb/c mouse model of asthma. Lung Breath. 2019;3(1):1–6. [Google Scholar]
- 18. Muehlmann LA, Zanatta AL, Farias CL, Bieberbach EW, Mazzonetto AC, Michellotto PV, Jr, Fernandes LC, Nishiyama A. Dietary supplementation with soybean lecithin increases pulmonary PAF bioactivity in asthmatic rats. J Nutr Biochem. 2010;21(6):532–537. [DOI] [PubMed] [Google Scholar]
- 19. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. [DOI] [PubMed] [Google Scholar]
- 20. Firinci F, Karaman M, Baran Y, Bagriyanik A, Ayyildiz ZA, Kiray M, Kozanoglu I, Yilmaz O, Uzuner N, Karaman O. Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol. 2011;11(8):1120–1126. [DOI] [PubMed] [Google Scholar]
- 21. Conrad ML, Yildirim AO, Sonar SS, Kiliç A, Sudowe S, Lunow M, Teich R, Renz H, Garn H. Comparison of adjuvant and adjuvant-free murine experimental asthma models. Clin Exp Allergy. 2009;39(8):1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aun MV, Bonamichi-Santos R, Arantes-Costa FM, Kalil J, Giavina-Bianchi P. Animal models of asthma: utility and limitations. J Asthma Allergy. 2017;10:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie J, Xi Y, Zhang Q, Chen G, Wei L, Lai K, Zhong N. An intratracheal challenge murine model of asthma: can bronchial inflammation affect the nose? Allergy Asthma Immunol Res. 2015;7(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1(4-5):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spaziano G, Urbanek K, Angelis A, Piegari E, Matteis M, Sgambato M, Tartaglione G, Russo TP, Cappetta D, Esposito G, Russo R, et al. Intratracheal administration of bone marrow-derived mesenchymal stem cells ameliorates lung function. Eur RespirJ. 2016;48:1–10.27365500 [Google Scholar]
- 26. Urbanek K, De Angelis A, Spaziano G, Piegari E, Matteis M, Cappetta D, Esposito G, Russo R, Tartaglione G, Palma R, Rossi F, et al. Intratracheal administration of bone marrow-derived mesenchymal stem cells ameliorates lung functionIntratracheal administration of bone marrow-derived mesenchymal stem cells ameliorates lung function. PloS One. 2016;11(7):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogulur I, Gurhan G, Aksoy A, Duruksu G, Inci C, Filinte D, Kombak FE, Karaoz E, Akkoc T. Suppressive effect of compact bone-derived mesenchymal stem cells on chronic airway remodeling in murine model of asthma. Int Immunopharmacol. 2014;20(1):101–109. [DOI] [PubMed] [Google Scholar]
- 28. Kurtz A. Mesenchymal stem cell delivery routes and fate. Int J Stem Cells. 2008;1(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jung JW, Kwon M, Choi JC, Shin JW, Park IW, Choi BW, Kim JY. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med J. 2013;54(5):1293–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tatsumi K, Ohashi K, Matsubara Y, Kohori A, Ohno T, Kakidachi H, Horii A, Kanegae K, Utoh R, Iwata T, Okano T. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun. 2013;431(2):203–209. [DOI] [PubMed] [Google Scholar]
- 31. Bonfield TL, Nolan Koloze MT, Lennon DP, Caplan AI. Defining human mesenchymal stem cell efficacy in vivo. J Inflamm. 2010;7(51):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martínez-González I, Cruz MJ, Moreno R, Morell F, Muñoz X, Aran JM. Human mesenchymal stem cells resolve airway inflammation, hyperreactivity, and histopathology in a mouse model of occupational asthma. Stem Cells Dev. 2014;23(19):2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Vyve T, Chanez P, Bernard A, Bousquet J, Godard P, Lauwerijs R, Sibille Y. Protein content in bronchoalveolar lavage fluid of patients with asthma and control subjects. J Allergy Clin Immunol. 1995;95(1 pt 1):60–68. [DOI] [PubMed] [Google Scholar]
- 34. Hamsten C, Wiklundh E, Grönlund H, Schwenk JM, Uhlén M, Eklund A, Nilsson P, Grunewald J, Häggmark-Månberg A. Elevated levels of FN1 and CCL2 in bronchoalveolar lavage fluid from sarcoidosis patients. Respir Res. 2016;17(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu Q-L, Chen Z. Establishment of different experimental asthma models in mice. Exp Ther Med. 2018;15(3):2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ou-Yang HF, Huang Y, Hu XB, Wu CG. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med. 2011;236(12):1461–1467. [DOI] [PubMed] [Google Scholar]
- 37. Cho KS, Park MK, Kang SA, Park HY, Hong SL, Park H, Yu HS, Roh HJ. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediators Inflamm. 2014;2014:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Groot JC, Ten Brinke A, Bel EH. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. 2015;1(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mohammadian M, Sadeghipour HR, Kashani IR, Jahromi GP, Omidi A, Nejad AK, Golchoobian R, Boskabady MH. Evaluation of simvastatin and bone marrow-derived mesenchymal stem cell combination therapy on airway remodeling in a mouse asthma model. Lung. 2016;194(5):777–785. [DOI] [PubMed] [Google Scholar]
- 40. Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533(1-3):222–239. [DOI] [PubMed] [Google Scholar]
- 41. Malaquias MAS, Oyama LA, Jericó PC, Costa I, Padilha G, Nagashima S, Lopes-Pacheco M, Rebelatto CLK, Michelotto PV, Xisto DG, Brofman PRS, et al. Effects of mesenchymal stromal cells play a role the oxidant/antioxidant balance in a murine model of asthma. Allergol Immunopathol. 2018;46(2):136–143. [DOI] [PubMed] [Google Scholar]
- 42. Reis AC, Alessandri AL, Athayde RM, Perez DA, Vago JP, Ávila TV, Ferreira TP, de Arantes AC, Coutinho DdeS, Rachid MA, Sousa LP, et al. Induction of eosinophil apoptosis by hydrogen peroxide promotes the resolution of allergic inflammation. Cell Death Dis. 2015;6(2):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karo-Atar D, Bitton A, Benhar I, Munitz A. Therapeutic targeting of the interleukin-4/interleukin-13 signaling pathway: in allergy and beyond. BioDrugs. 2018;32(3):201–220. [DOI] [PubMed] [Google Scholar]
- 44. Kaur D, Gomez E, Doe C, Berair R, Woodman L, Saunders R, Hollins F, Rose FR, Amrani Y, May R, Kearley J, et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: airway smooth muscle crosstalk. Allergy. 2015;70(5):556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol. 2014;205(5):621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ijpma G, Panariti A, Lauzon AM, Martin JG. Directional preference of airway smooth muscle mass increase in human asthmatic airways. Am J Physiol Lung Cell Mol Physiol. 2017;312(6):L845–L854. [DOI] [PubMed] [Google Scholar]
- 47. Corren J. Role of interleukin-13 in asthma. Curr Allergy Asthma Rep. 2013;13(5):415–420. [DOI] [PubMed] [Google Scholar]
- 48. Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134(3):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gon Y., Hashimoto S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int. 2018;67(1):12–17. [DOI] [PubMed] [Google Scholar]
- 50. Yao Y, Fan XL, Jiang D, Zhang Y, Li X, Xu ZB, Fang SB, Chiu S, Tse HF, Lian Q, Fu QL. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Reports. 2018;11(5):1120–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Curran DR, Cohn L. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am J Respir Cell Mol Biol. 2010;42(3):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cohn L, Whittaker L, Niu N, Homer RJ. Cytokine regulation of mucus production in a model of allergic asthma. Novartis Found Symp. 2002;248:201–220. [PubMed] [Google Scholar]
- 53. Chenuet P, Fauconnier L, Madouri F, Marchiol T, Rouxel N, Ledru A, Mauny P, Lory R, Uttenhove C, van Snick J, Iwakura Y, et al. Neutralization of either IL-17A or IL-17F is sufficient to inhibit house dust mite induced allergic asthma in mice. Clin Sci. 2017;131(20):2533–2548. [DOI] [PubMed] [Google Scholar]
- 54. Dai R, Yu Y, Yan G, Hou X, Ni Y, Shi G. Intratracheal administration of adipose derived mesenchymal stem cells alleviates chronic asthma in a mouse model. BMC Pulm Med. 2018;18(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108(3):430–438. [DOI] [PubMed] [Google Scholar]
- 56. Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, Li D, Zhang G, Huang B, Feng ZH. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181(9):6117–6124. [DOI] [PubMed] [Google Scholar]
- 57. Dias PM, Banerjee G. The role of TH17/IL-17 on eosinophilic inflammation. J Autoimmun. 2013(1);40:9–20. [DOI] [PubMed] [Google Scholar]
- 58. Coomes SM, Kannan Y, Pelly VS, Entwistle LJ, Guidi R, Perez-Lloret J, Nikolov N, Müller W, Wilson MS. CD4+ Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol. 2017;10(1):150–161. [DOI] [PubMed] [Google Scholar]
- 59. Song SW, Kim KE, Choi JW, Lee CY, Lee J, Seo HH, Lim KH, Lim S, Lee S, Kim SW, Hwang KC. Proteomic analysis and identification of paracrine factors in mesenchymal stem cell-conditioned media under hypoxia. Cell Physiol Biochem. 2016;40(1-2):400–410. [DOI] [PubMed] [Google Scholar]
- 60. Caplan AI. There is no “Stem cell mess”. Tissue Eng Part B Rev. 2019;25(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Keyhanmanesh R, Rahbarghazi R, Ahmadi M. Systemic transplantation of mesenchymal stem cells modulates endothelial cell adhesion molecules induced by ovalbumin in rat model of asthma. Inflammation. 2018;41(6):2236–2245. [DOI] [PubMed] [Google Scholar]
- 62. Keyhanmanesh R, Rahbarghazi R, Aslani MR, Hassanpour M, Ahmadi M. Systemic delivery of mesenchymal stem cells condition media in repeated doses acts as magic bullets in restoring IFN-γ/IL-4 balance in asthmatic rats. Life Sci. 2018;212:30–36. [DOI] [PubMed] [Google Scholar]