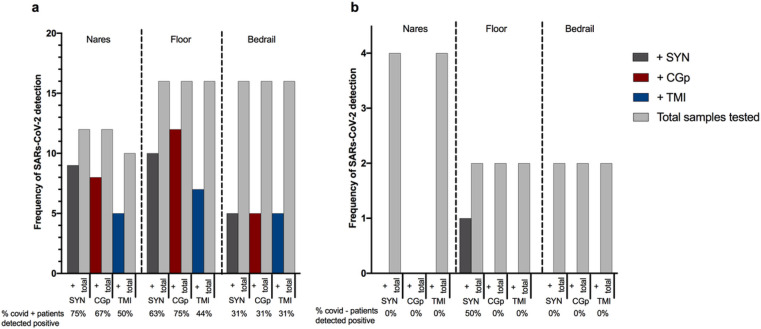
Figure 3.
Comparison of CDC approved SYN swabs, consumer-grade CGp and bulk TMI swab congruence compared to clinical-grade hospital tests using synthetic-tipped plastic shafted NP swabs for twenty participants in the clinical setting. a) SARs-CoV-2 positive patients (n=16) sampled with three swab types across three environments: nares, floor, and bedrail. ‘+’ samples (dark grey = SYN, red = CGp, blue = TMI) refer to samples which tested positive for SARs-CoV-2 out of the total samples tested for that particular swab type (light grey bar). Percentage of positive tests per swab type are below x axis for each environmental sample. b) SARs-CoV-2 negative patients (n=4) with three swab types across three environments: nares, floor, and bedrail. Same nomenclature as above.