Figure 1: The modus operandi of CD4+ FOXP3+ CD25high Tregs:
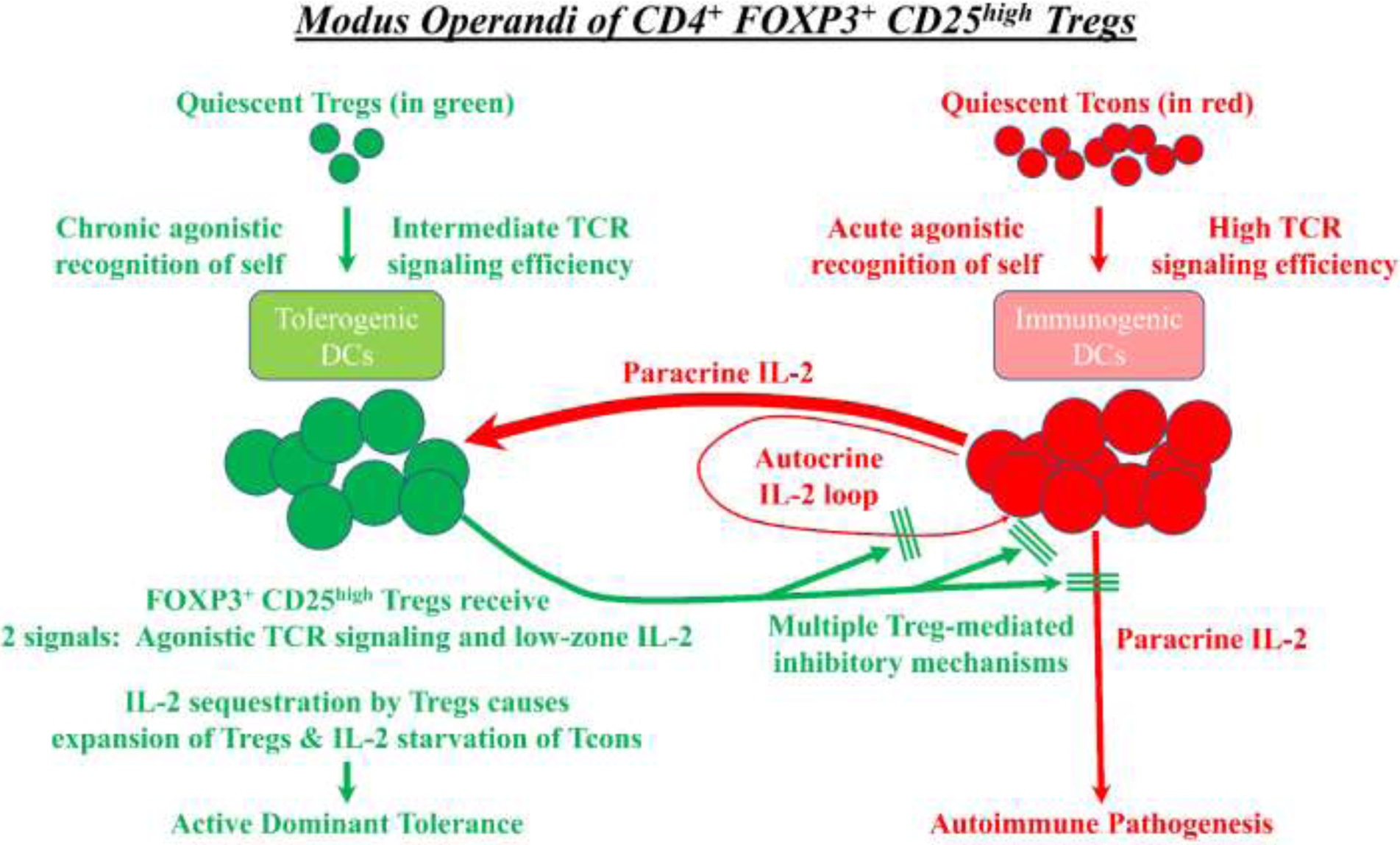
The defining hallmark of FOXP3+ Tregs is the functional coupling of TCR specificity for agonistic self, chronic activation in response to self, and suppression of Tcon-mediated reactions against self. Quiescent Tregs (green) are selected in the thymus and periphery to recognize self-MHC ligands as intermediate-efficiency agonistic ligands, which is postulated to maintain viability of FOXP3+ Tregs and elicit high surface expression of CD25. Rogue Tcons (red) that have the potential to mediate autoimmune pathogenesis recognize agonistic self-MHC ligands and produce IL-2, which is a source of paracrine IL-2 for Tregs. In this environment, Tregs receive two signals including agonistic TCR ligation and paracrine IL-2 stimulation. Due to their superlative CD25 expression, Tregs monopolize the local IL-2 supply to drive Treg growth while depriving Tcons of the IL-2 needed for effector function and survival. Aside from sequestering IL-2, activated effector Tregs use multiple direct and indirect mechanisms to block Tcon-mediated autoimmune pathogenesis.
