Figure 6: Tolerogenic vaccination: bridging holes in the Treg repertoire to enhance bystander inhibition and infectious tolerance.
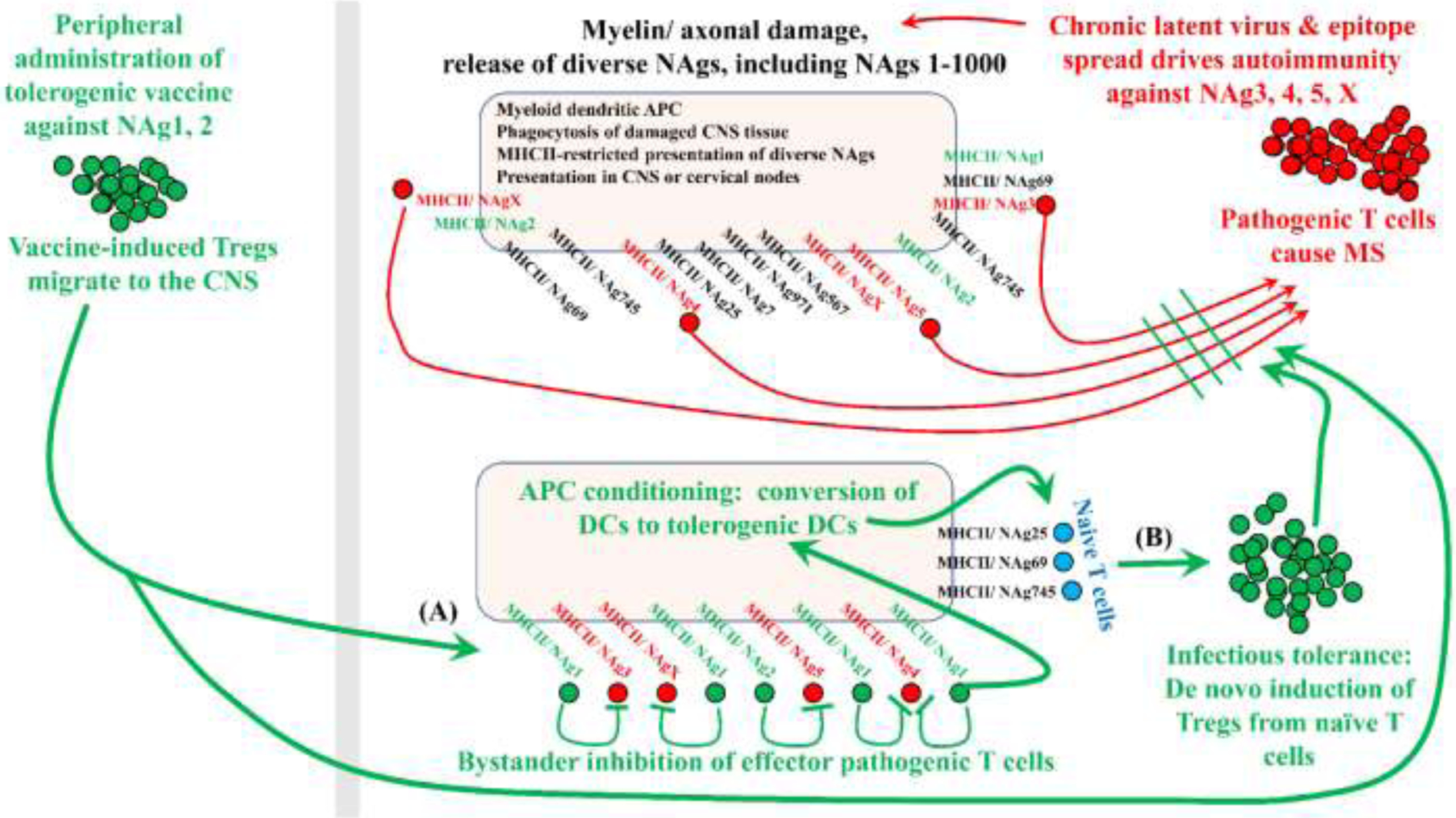
Tolerogenic vaccination must target pathogenic T cell clonotypes without knowledge of the pathogenic epitopes. For example, if pathogenesis in MS is driven by NAg3, 4, 5, and X, then CNS-derived DCs will present these pathogenic NAgs together with myriads of other NAgs because CNS tissue destruction releases diverse NAgs including endogenous versions of the vaccine NAgs. As a result, local DCs will present diverse NAgs including the unknown pathogenic NAgs and the known vaccine-specific NAgs (i.e., not derived from the vaccine per se but derived from damaged myelin). Upon tolerogenic vaccination with nonpathogenic NAg1 and NAg2, peripheral DCs will drive expansion of NAg1/2-specific Tregs, which will migrate throughout the body and home into tissues bearing high endogenous concentrations of NAg1 and NAg2. DCs presenting damage-associated CNS NAg3, 4, 5, and X represent the interface of vaccine-generated Tregs with pathogenic Tcons. This interface is the checkpoint that re-establishes tolerance (A) by reinforcing bystander inhibition of pathogenic Tcons and (B) by eliciting infectious tolerance via de novo differentiation of naïve tissue-resident Tcons into Tregs.
