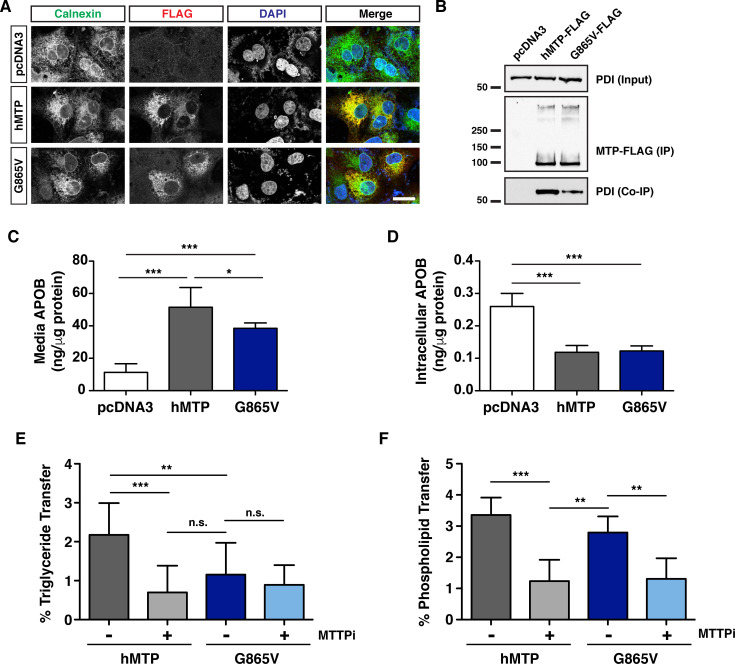
Fig 6. The corresponding c655 mutation in human MTTP disrupts TG transfer but not PL transfer activity.
(A) Immunofluorescence in COS-7 cells expressing wild-type human MTTP-FLAG or human MTTP(G865V)-FLAG proteins using anti-FLAG (red) and anti-Calnexin (green) antibodies; scale = 25 μm. (B) Human MTP-FLAG proteins (WT and G865V) were immunoprecipitated from COS-7 cell lysate (400 μg protein) using the M2 flag antibody and immunoblots were probed for both FLAG and PDI. For input, 15 μg protein was used. (C, D) COS-7 cells were co-transfected with human APOB48 and either wild-type human MTTP-FLAG, MTTP(G865V)-FLAG or empty pcDNA3 plasmids. After 72 h, APOB48 was measured via ELISA in media (C) or in the cell (D). Data are representative of 7 independent experiments (each data point is the mean of three technical replicates), pcDNA3 control data is re-graphed from Fig 5A & 5B (data for Figs 5A, 5B, 6C and 6D were generated together); mean +/- SD, One-Way ANOVA with Bonferroni post-hoc tests, * p < 0.05, *** p < 0.001. (E) COS-7 cells were transfected with plasmids expressing human wild-type or MTTP(G865V)-FLAG constructs. Cells were lysed and 60 μg of protein was used to measure TG transfer activity in the presence or absence of the MTP inhibitor lomitapide (MTTPi, 1 μM) (% after 45 min); n = 3 (each n is the mean of three technical replicates from independent experiments), mean +/- SD, One-way ANOVA with Bonferroni post-hoc tests, ** p < 0.01, ***p < 0.001, n.s. not significant). (F) Wild-type and mutant MTP proteins were purified using anti-FLAG antibodies and used to measure PL transfer in the presence or absence of lomitapide (MTTPi, 1 μM) (180 min); n = 3 (each n is the mean of three technical replicates from independent experiments), mean +/- SD, randomized block ANOVA with Bonferroni post-hoc tests, ** p < 0.01, ***p < 0.001.