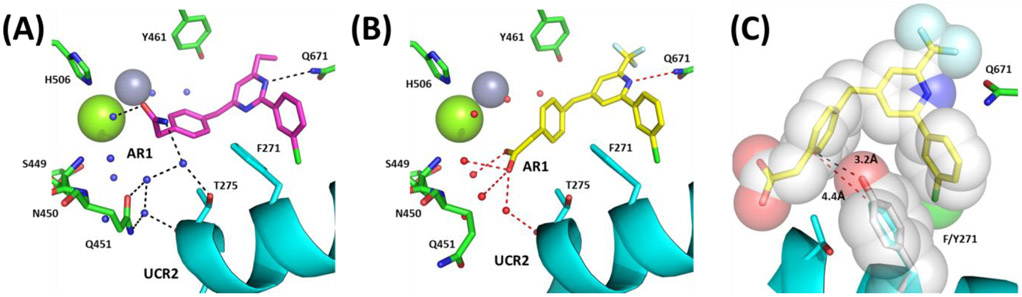
Figure 7:
Comparison and selectivity of 23 and 28: (A) 23 with AR1 amide pointing up in the pocket to form water-mediated hydrogen bonds with the main-chain and side-chain of UCR2 (cyan) threonine275 (T275) and catalytic domain glutamine451 (Q451), histidine506 (H506) and waters surrounding the magnesium atom (green sphere). (B) 28 (PDB: 6NJJ) with AR1 carboxylic acid pointing down losing water-mediated hydrogen bonds with histidine506 (H506) and the side-chain of threonine275 (T275), but gaining water-mediated hydrogen bonds to serine449 (S449), asparagine450 (N450), and the main-chain of glutamine451 (Q451), while maintaining the main-chain interaction with UCR2 threonine275 (T275). (C) Modeling of tyrosine271 (Y271) in place of phenylalanine271 (Y271) onto the crystal structure of PDE4D and 28. The closest approach by phenylalanine271 to 28 is 4.4Å which is reduced with tyrosine to 3.2Å.