Table 1:
Inhibitory activity of triazine Ar1 substitution
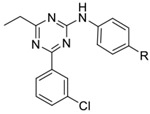 |
||||||
|---|---|---|---|---|---|---|
| Compound | R | PDE4D7- S129D IC50 (nM)a |
PDE4B1- S133D IC50 (nM)b |
PDE4D7- S129(wt) IC50 (nM)c |
Ratio B/Dd |
Ratio Basal/Activatede |
| 4 | −(CH2)3-OH | 0.2 | 11 | 27 | 55 | 135 |
| 5 | −CH2CH2OH | 0.5 | 24 | 102 | 48 | 204 |
| 6 | −CH2CONH2 | 5 | 48 | 203 | 10 | 41 |
| 7 | −CH2OH | 12 | 105 | 351 | 9 | 30 |
| 8 | −CONH2 | 22 | 44 | 252 | 2 | 11 |
| 9 | −CH(Me)CO2H | 96 | 1090 | NCf | 11 | |
| 10 | −CO2H | 213 | 241 | 482 | 1 | 2 |
| 11 | −CH2CO2H | 681 | 781 | 4040 | 1 | 6 |
PDE4D7-S129D is a dimeric isoform of PDE4D that contains a UCR1 mutation (S129D) that mimics PKA phosphorylation.
PDE4B1-S133D is a dimeric isoform of PDE4B that also contains a UCR1 mutation (S133D) that mimics PKA phosphorylation.
PDE4D7-S129(wt) is the native isoform of PDE4D7 that lacks the UCR1 phosphorylation mimetic mutation.
B/D is the ratio of IC50 for PDE4B1-S133D/PDE4D7-S129D.
Basal/Activated is the ratio of IC50 for PDE4D7-S129(wt)/PDE4D7-S129D.
NC -Not calculated as IC50 >10,000 nM. See experimental section for methods and detailed synthetic procedures.
