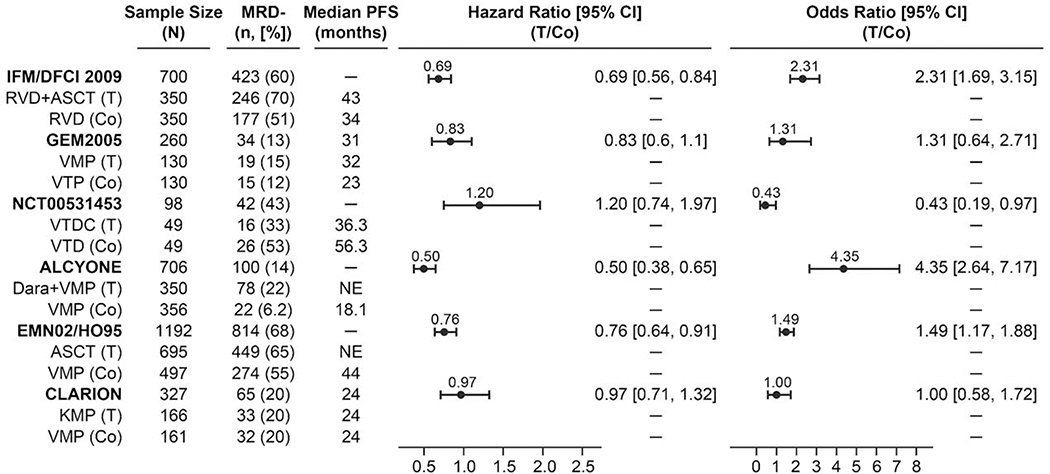
Figure 2.
Treatment effect on progression-free survival and minimal residual disease for randomized studies using multiparameter flow cytometry, next-generation flow, and next-generation sequencing. MRD-negativity rates were calculated based on sample size and patients that were excluded from MRD assessment were MRD positive.
ASCT, autologous stem cell transplant; Co, control; CI, confidence interval; Dara, daratumumab; KMP, carfilzomib, melphalan, and prednisone; MRD, minimal residual disease; PFS, progression-free survival; RVD, lenalidomide, bortezomib, and dexamethasone; T, treatment; VMP, bortezomib, melphalan, and prednisone; VTD, bortezomib, thalidomide, and dexamethasone; VTDC, bortezomib, thalidomide, dexamethasone, and cyclophosphamide; VTP, bortezomib, thalidomide, and prednisone.