FIGURE 1.
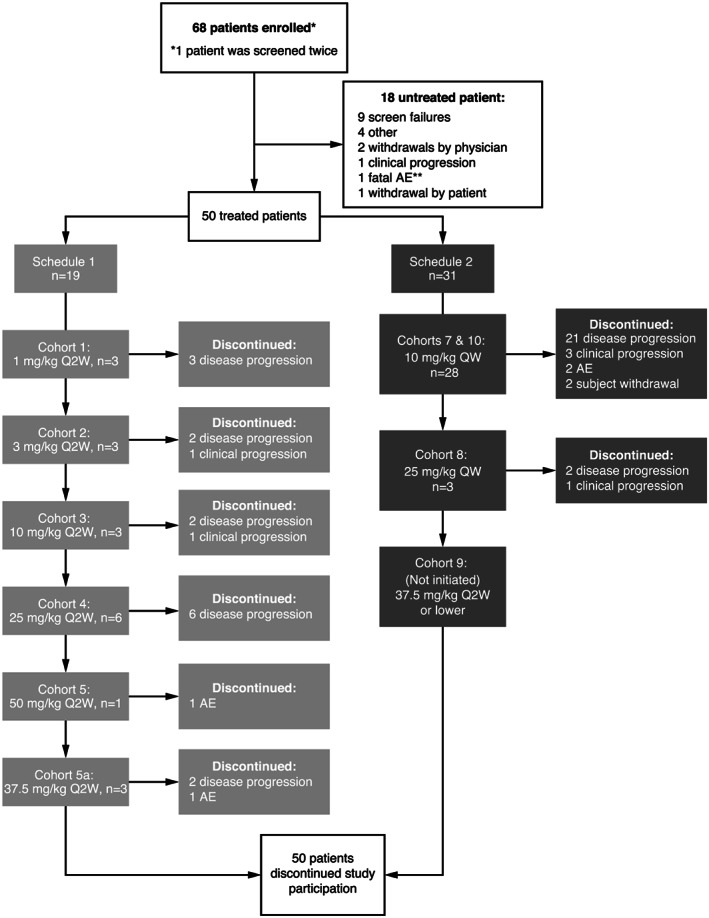
Disposition of patients in both schedules and all cohorts of the phase 1 imalumab study. Enrolled patients were those who had signed an informed consent form. AE, adverse event; Q2W, every two weeks; QW, every week

Disposition of patients in both schedules and all cohorts of the phase 1 imalumab study. Enrolled patients were those who had signed an informed consent form. AE, adverse event; Q2W, every two weeks; QW, every week