FIGURE 2.
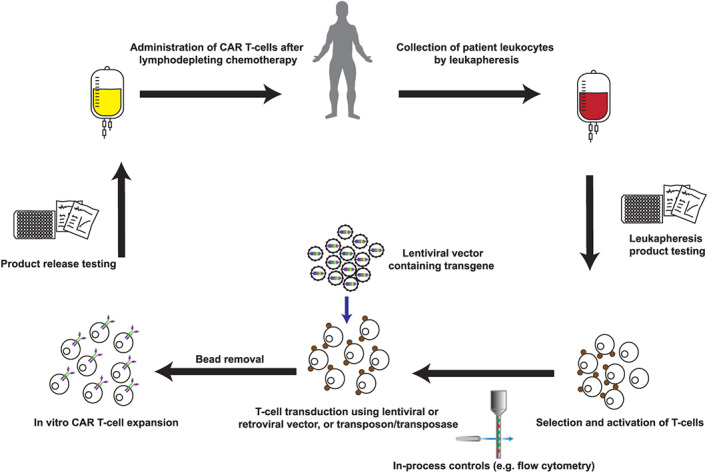
Manufacture of chimeric antigen receptor (CAR) T‐cells. Manufacture of autologous CAR T‐cells typically begins with patient blood leucocytes obtained by leukapheresis. A sample of this input product is sent for screening tests, while T‐cells are purified and activated using immunomagnetic beads or plate‐bound antibodies. In‐process controls may include purity checks by flow cytometry. A transgene is introduced using a lentiviral or retroviral vector, or a transposon/transposase system. CAR T‐cells are expanded in vitro using optimised cell culture conditions and in the presence of cytokines. Product release testing is required before CAR T‐cells are released for administration to the patient, typically following lymphodepleting chemotherapy
