FIGURE 4.
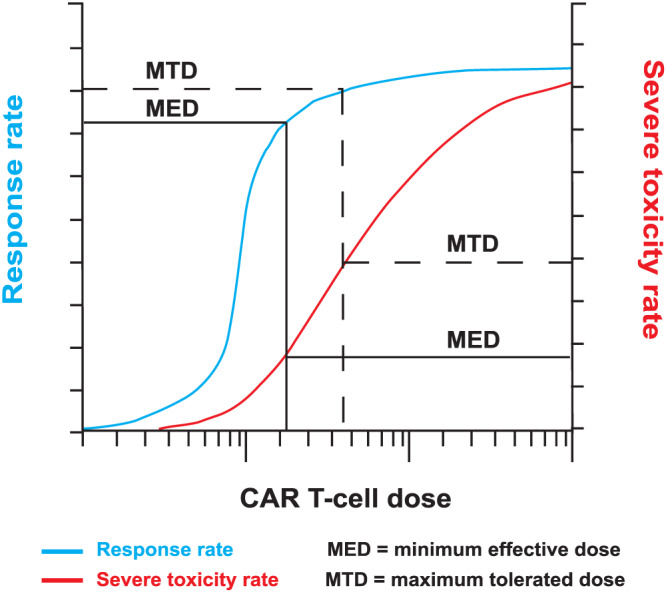
Selecting an optimal chimeric antigen receptor (CAR) T‐cell dose. Beneath a certain dose threshold, CAR T‐cells may fail to expand within the recipient, and both response and severe toxicity are unlikely. Above this threshold, clinical efficacy may increase rapidly until a minimum effective dose is reached, beyond which there may be little further improvement in response rate with increasing dose. In contrast, the rate of severe toxicities may continue to rise as the dose is increased. Phase I trial designs that seek to determine the minimum effective dose as well as the maximum tolerated dose may facilitate selection of a dose that maximises response rate without undue toxicity risk
