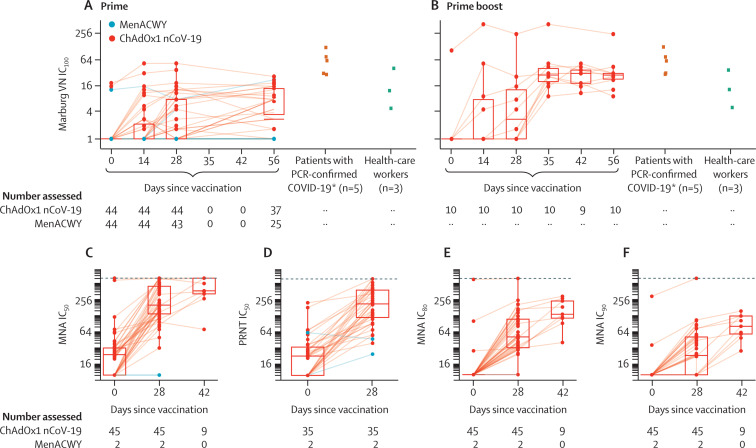
Figure 4.
Live SARS-CoV-2 neutralisation assays (Marburg VN and PHE PRNT50) and microneutralisation assays (PHE MNA)
Panels A and B show live SARS-CoV-2 neutralisation (Marburg VN) in prime (A) and prime boost (B) trial participants (boosted at day 28) and convalescent plasma from patients with PCR-confirmed COVID-19 and asymptomatic health-care workers. Panels C, E, and F show the PHE MNA (at IC50, IC80, and IC90, respectively) and panel D the PHE PRNT50. The day 42 timepoint was only measured in participants who received a booster dose at day 28. Solid lines connect samples from the same participant. Boxes show median (IQR). Dotted lines show upper limits of detection. MenACWY=meningococcal group A, C, W-135, and Y conjugate vaccine. PHE=Public Health England. MNA=microneutralisation assay. PRNT=plaque reduction neutralisation test. VN=virus neutralisation. IC=inhibitory concentration. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. *ELISA results for these five convalescent plasma samples are shown in figure 3 as red stars.