Figure 4.
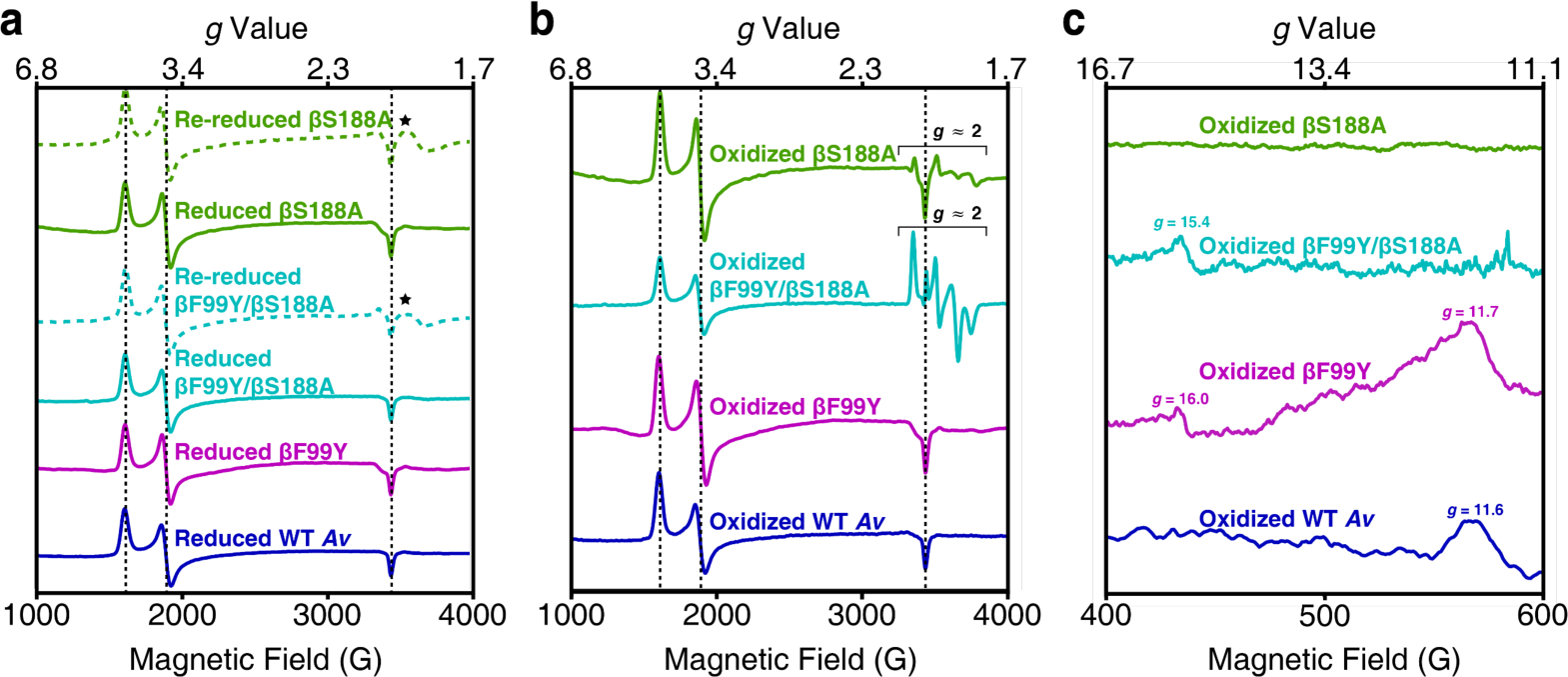
X-band EPR spectra of the Av MoFeP variants. All spectra were collected at 5–10 K. Dashed black lines mark the features that arise from the S = 3/2 signal associated with FeMoco. (a) Perpendicular-mode EPR spectra of DT-reduced and DT-re-reduced MoFeP. Reduced mutants have the same FeMoco-associated features present in wild-type Av. The spectra of re-reduced βS188A and βF99Y/βS188A MoFeP display new features in the g ≈ 1.9 region (⋆) that may arise from some amount of degraded protein. The data (from top to bottom) were collected at 8 K, 6 K, 9 K, 5 K, 6 K, and 8 K. (b) Perpendicular-mode EPR spectra of IDS-oxidized MoFeP. The spectra of βS188A and βF99Y/βS188A MoFeP have features in the g ≈ 2.0 region attributed to a population of MoFeP containing only one Fe3+ per P-cluster. Data (from top to bottom) were collected at 5 K, 9 K, 6 K, and 8 K. (c) Parallel-mode spectra of the oxidized Av MoFeP demonstrate that ligation of the oxygenic ligand to the P-cluster results in an integer spin. Tyrosine ligation (βF99Y/βS188A and βF99Y) results in features at g ≈ 16, and serine ligation (βF99Y and wild-type) at g ≈ 12.
