Abstract
Study Objectives:
Black individuals are disproportionately affected by diabetes, cardiovascular disease, obesity, and OSA. Adherence to PAP therapy has been reported to be lower among black individuals. This study seeks to examine associations between black race and PAP adherence among veterans with OSA.
Methods:
This was a retrospective study. Veterans newly diagnosed with OSA at a single Department of Veterans Affairs sleep center who were prescribed a modem-enabled PAP device between January 2015 and November 2017 were enrolled. PAP adherence was defined as ≥ 4 hours nightly usage for at least 70% of nights measured at 30 days from PAP setup. We examined the relationship between race and adherence, controlling for sex, marital status, age, socioeconomic status, residual apnea-hypopnea index), and mask leak.
Results:
Of 3013 patients identified with OSA, 2571 (85%) were newly started on PAP therapy (95% male, aged 59 years ± 14 years, 45% married, 8% with neighborhood socioeconomic disadvantage). Twenty-five percent of participants were black, and 57% were white. PAP adherence at 30 days was 50% overall (42% among blacks, 53% among nonblacks). Black race was associated with reduced 30-day PAP adherence in unadjusted (P < .001) and adjusted logistic regression models (odds ratio = 0.64; 95% CI, 0.53 – 0.78; P < .001).
Conclusions:
Among veterans with OSA, black race was associated with reduced PAP adherence. These findings suggest health inequality among black individuals in the treatment of OSA.
Citation:
Hsu N, Zeidler MR, Ryden AM, Fung CH. Racial disparities in positive airway pressure therapy adherence among veterans with obstructive sleep apnea. J Clin Sleep Med. 2020;16(8):1249–1254.
Keywords: obstructive sleep apnea, patient adherence, race, veterans
BRIEF SUMMARY
Current Knowledge/Study Rationale: Race is a known contributor to health disparities. Prior studies examining the relationship between black race and positive airway pressure adherence looked at small cohorts of patients or used single-measure proxies for socioeconomic status (ie, zip code, education level, or household income).
Study Impact: This study is the largest to date that examines the relationship between black race and positive airway pressure adherence in the treatment of obstructive sleep apnea, while utilizing objective cloud-based adherence data and adjusting for neighborhood socioeconomic disadvantage based on multiple domains, including income, education, employment, and housing quality. This study provides strong evidence of health inequality among black veterans with obstructive sleep apnea.
INTRODUCTION
OSA is a chronic disease that causes intermittent hypoxia and sleep fragmentation.1 Untreated OSA has been associated with an increased risk for automobile or workplace accidents, hypertension, diabetes, and cardiovascular events such as stroke or myocardial infarction.2–7 The prevalence of OSA has been documented to be up to 69% in certain cohorts of veterans, who overall comprise nearly 10% of the American population.8–10 Positive airway pressure (PAP) is the first-line treatment for OSA.1 Clinical studies and insurance companies generally define PAP adherence as a minimum of 4 hours of nightly usage for 70% of nights during a consecutive 30-day period.11 Yet, adherence to PAP therapy for OSA remains poor. Over 15% of patients discontinue treatment after a few nights of use, and approximately 50% abandon therapy within 1 year.12–15
Many factors have been correlated with PAP usage, including but not limited to race, sex, marital status, age, socioeconomic status, residual AHI, and mask leak.16–22 Black individuals with OSA may have reduced PAP adherence, but prior studies examining this relationship have been limited by small sample sizes, reliance on self-reported adherence, or lack of full adjustment for potential confounders such as socioeconomic status.16,22–29
Detecting racial health inequalities in PAP therapy is an important first step in reducing health disparities among individuals with OSA. In a conceptual framework developed by Department of Veterans Affairs researchers, the three phases of disparities research are (1) detecting (eg, define vulnerable populations, measure disparities in vulnerable populations, consider confounding factors), (2) understanding (eg, identify determinants of health disparities at the level of patients, provider, clinical encounter, and health care system), and (3) reducing (ie, intervene, evaluate, translate and disseminate, and change policy).30
As an initial step toward reducing health disparities, we sought to detect racial health inequalities at our institution. With the large-scale use in our sleep center of modem-enabled PAP devices that transmit objective PAP adherence data to cloud-based servers, we were provided a unique opportunity to evaluate patterns of PAP use in a large and racially diverse veteran population within the context of a closed medical system. We hypothesized a priori that black race would be associated with worse PAP adherence at 30-day follow-up among veterans with OSA.
METHODS
Overall design and data sources
This was a retrospective cohort study utilizing PAP therapy information stored in a cloud-based database and patient records housed within the Veterans Health Administration Corporate Data Warehouse, which is a secure collection of databases containing demographic, clinical, and utilization information that can be accessed via the Veterans Informatics and Computing Infrastructure. Local institutional review board approval was obtained (PCC 2018-100999).
Participants
We included individuals aged ≥ 18 years who had been diagnosed with OSA via home sleep apnea testing or in-lab polysomnography and were newly setup with modem-enabled PAP therapy between January 1, 2015 and December 31, 2017 at the Veterans Affairs Greater Los Angeles Healthcare System. Patients who had previously received PAP therapy were excluded from the study. Veterans referred for a sleep study at our institution were screened by sleep clinicians for contraindications to home sleep apnea testing in accordance with American Academy of Sleep Medicine guidelines, and those with contraindications to home sleep apnea testing underwent attended polysomnography. Obstructive sleep apnea was diagnosed based on American Academy of Sleep Medicine 1A guidelines. Each veteran with OSA was contacted by a sleep clinician about the OSA diagnosis and was offered a next-available appointment for PAP device setup. A Philips Respironics DreamStation Auto CPAP (initial settings typically 6–16 cm H2O) was issued, and education about PAP use was provided by a trained respiratory therapist during a standardized face-to-face visit.
Measures
The primary outcome was adherence at 30 days following PAP initiation and was defined as ≥ 4 hours of PAP use on 70% of nights in a consecutive 30-day period. A 30-day window prevented the introduction of confounders, specifically telemedicine visits by our sleep therapy–trained respiratory therapists, which were scheduled after this time period to evaluate adherence and support veteran PAP use. The primary predictor, black race, was extracted from the Corporate Data Warehouse.
We identified, based on the published literature, relevant covariates that could confound the relationship between black race and PAP adherence.16–22 Device-calculated parameters (eg, minutes of usage, residual AHI, and leak) were exported from a cloud database that stored the patient’s nightly therapy data. Additional demographic and clinical information (eg, age, sex, marital status) were also obtained from the Corporate Data Warehouse.
Neighborhood socioeconomic status was estimated using the area deprivation index (ADI) as a surrogate measure. Single measures of socioeconomic status suffer from methodological issues such as unreliability, difficulty with data collection, variation over a lifetime, inadequate classification of unemployed or retired individuals, and lack of concordance between individual measures (eg, income and education).31 Therefore, composite measures may be more appropriate and accurate in capturing socioeconomic status.32 Due to the retrospective nature of this study, comprehensive individual-level factors could not be ascertained and therefore a validated neighborhood-level composite index was used. The ADI is a publicly available composite score generated from 17 socioeconomic variables collected in the 2013 American Community Survey and measures neighborhood disadvantage via social factors such as education, employment, income, and housing quality.33,34 The ADI has been shown previously to correlate with clinical outcomes such as chronic disease management success and hospital readmissions.35,36 Veteran mailing addresses were obtained via Veterans Informatics and Computing Infrastructure and were geocoded to the census block group (ie, neighborhood) level. The geocoded records were merged with the 2013 ADI data from the University of Wisconsin, School of Medicine and Public Health (www.neighborhoodatlas.medicine.wisc.edu) to obtain an ADI score for each address. Those in the first percentile are considered the least disadvantaged, and those in the 100th percentile are the most disadvantaged. The presence of neighborhood disadvantage was defined as the top 15% of ADI percentiles, based on prior analyses showing increased readmission risks at this cutoff.33
Statistical analyses
All statistical analyses were performed with RStudio (version 1.1.456; RStudio Inc, Boston, MA). Continuous variables were reported as mean plus or minus standard deviation (± SD). We compared demographic and clinical characteristics across groups using t tests, χ2 tests, and correlation coefficients, as appropriate. Multicollinearity was assessed using the Farrar-Glauber F-test and variance inflation factor. Multiple logistic regression models were fit with predetermined covariates: sex, marital status, age, socioeconomic status represented by ADI, mean residual AHI, and mean residual mask leak. We used an alpha level of .05 for all statistical tests.
RESULTS
A total of 3013 individuals were identified, of whom 2571 (85%) were newly started on PAP therapy. The demographic characteristics of the sample are presented in Table 1. The study population was predominantly middle-aged men (95% male, mean age 59 ± 14 years). Black veterans made up 25% of the study population. At 30 days following initial PAP setup, the overall adherence was 50% (n = 1289) with 242 ± 163 minutes (4.0 ± 2.7 hours) of nightly use. Compared to nonblack veterans, black veterans were younger with a lower likelihood of being married. Black veterans also had reduced 30-day PAP adherence (42% versus 53%) and nightly usage (216 ± 135 versus 251 ± 166 minutes). Additionally, reduced PAP adherence in black veterans was apparent as early as 1 week after treatment initiation (41% versus 51%, P < .001).
Table 1.
Participants’ characteristics.
| Variables | Study Population (n = 2571) | Black Race (n = 644) | Nonblack Race (n = 1927) | P-value |
|---|---|---|---|---|
| Male | 95% | 93% | 96% | < .01 |
| Marital Status | ||||
| Never married | 22% | 24% | 21% | .12 |
| Married | 45% | 35% | 49% | < .001 |
| Divorced | 30% | 39% | 26% | < .001 |
| Widowed | 2% | 2% | 2% | .63 |
| Unknown | 1% | .5% | 1% | .12 |
| Age (years) | 59 ± 14 | 61 ± 23 | 59 ± 15 | < .001 |
| Area deprivation index percentile (mean) | 33 | 33 | 33 | .99 |
| Residual AHI (events/h) | 4.7 ± 5.2 | 4.7 ± 26.3 | 4.8 ± 5.4 | .68 |
| Mask leak (liters/min) | 40.8 ± 23.9 | 41.0 ± 24.0 | 40.8 ± 24.2 | .88 |
| ≥ 4-h PAP use for ≥ 70% of days, at 7 days | 48% | 41% | 51% | < .001 |
| Average nightly use (min), at 7 days | 232 ± 169 | 207 ± 163 | 241 ± 171 | < .001 |
| ≥ 4-h PAP use for ≥ 70% of days, at 30 days | 50% | 42% | 53% | < .001 |
| Average nightly use (min), at 30 days | 242 ± 163 | 216 ± 135 | 251 ± 166 | < .001 |
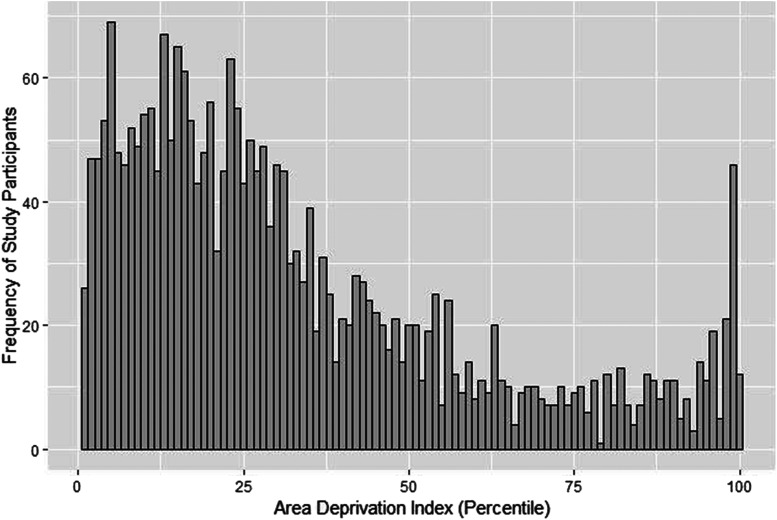
Figure 1 shows the distribution of neighborhood socioeconomic disadvantage. ADI percentiles ranged from 1% to 100%, with a mean of 33% and a median of 26%. Only 8% of the cohort resided in the most disadvantaged neighborhoods, based on the published cutoff that the most disadvantaged neighborhoods are represented by the top 15% national ADI.33
Figure 1. Distribution of area deprivation index percentiles among veterans with obstructive sleep apnea receiving care at the Veterans Affairs Greater Los Angeles Healthcare System.
Socioeconomic status was estimated by an area deprivation index, which ranks neighborhoods at the level of the census block-group and accounts for income, education, employment, and housing quality. Those in the first percentile are considered the least disadvantaged, and those in the 100th percentile are the most disadvantaged. The vast majority of study participants did not live in a disadvantaged neighborhood, based on the published cutoff that the most disadvantaged neighborhoods are represented by the top 15% national ADI.
Table 2 presents 30-day PAP adherence, stratified by the adherence factors. In univariate regression, PAP adherence was associated with race (black versus nonblack race), marital status (married state versus not married), age, neighborhood socioeconomic status (represented by ADI percentile), residual AHI, and mask leak. Although black veterans represented 25% of the total study population, they represented 21% of adherent and 29% of nonadherent patients (P < .001). In the adjusted logistic regression model, black race remained a significant predictor of reduced PAP adherence at 30 days (odds ratio = 0.64; 95% confidence interval, 0.53–0.78; P < .001).
Table 2.
Outcome of PAP adherence at one month (≥ 4h PAP use for ≥ 70% of days) in unadjusted and adjusted logistic regression models.
| Variables | Unadjusted Odds Ratioc [95% CI] | Unadjusted P-value | Adjusted Odds Ratioc [95% CI] | Adjusted P-value |
|---|---|---|---|---|
| Race | ||||
| Nonblack racea | Reference | Reference | ||
| Black race | 0.66 [0.55–0.79] | <.001 | 0.64 [0.53–0.78] | <.001 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 1.04 [.72–1.51] | .90 | 0.94 [0.63–1.39] | .66 |
| Marital status | ||||
| Not marriedb | Reference | Reference | ||
| Married | 1.61 [1.38–1.89] | <.001 | 1.39 [1.18–1.64] | <.001 |
| Age | 1.01 [1.01–1.02] | <.001 | 1.02 [1.02–1.03] | <.001 |
| Area deprivation index percentile | 1.00 [1.00–1.01] | .02 | 1.00 [1.00–1.01] | .03 |
| Residual AHI (events/h) | 0.96 [0.95–0.98] | <.001 | 0.97 [0.94–0.97] | <.001 |
| Mask leak (liters/min) | 0.98 [0.97–0.98] | <.001 | 0.98 [0.97–0.98] | <.001 |
a Includes White, Asian, Native Hawaiian/Pacific Islander, American Indian, Not Stated. b Includes Never Married, Divorced, Widowed, Unknown. c Odds ratios for continuous variables (age, area deprivation index percentile, AHI, and mask leak) can be interpreted as the change in the odds of 30-day PAP adherence for every unit increase in the variable. Odds ratios for categorical variables (black race, male sex, married state, and 7-day adherence) represent the change in odds of 30-day PAP adherence if the variable were true. CI = confidence interval.
There was no significant difference in PAP adherence with respect to other racial subgroups (eg, Asian, Hawaiian/Pacific Islander, and American Indian) (Table S1 in the supplemental material).
DISCUSSION
To our knowledge, this is the largest real-world analysis of the relationship between race and PAP adherence that controls for socioeconomic status and utilizes objective measures of PAP adherence. Among 2571 veterans with OSA who were newly started on PAP therapy, we found reduced PAP adherence among individuals who self-reported black race. Even after adjusting for potential confounders such as neighborhood socioeconomic disadvantage, black race was associated with a 36% reduction in PAP adherence.
The significant correlation between black race and PAP adherence that was seen in our large cohort is supported by other studies. Among patients diagnosed with OSA during preoperative screening or following inpatient hospitalization for decompensated congestive heart failure, CPAP adherence was reduced among black individuals; however, socioeconomic factors were not assessed.24,27 At the Miami Veterans Affairs sleep clinic, black individuals had 1 hour less of nightly PAP use compared to whites when adjusted for education level.28 Similarly, in another study, reduced adherence was seen among African American veterans, although zip code (specifically median household income for zip code) was used as the measure of socioeconomic status.37 A randomized controlled trial across 5 cities in the United States of home versus lab-based diagnosis of OSA found that both black race and lower economic status (again based on zip code) predicted poor PAP adherence, but zip codes can encompass a variety of neighborhoods with different socioeconomic statuses.16
Our study’s findings are strengthened by our use of a large data set inclusive of all veterans receiving modem-enabled PAP therapy within our enrollment window. Device parameters were directly obtained from a secure cloud-based system, which provided more accurate and complete usage assessments than did prior studies in which individuals self-reported adherence or whose data were lost along with follow-up clinic visits. In contrast with other large-scale retrospective analyses in which adherence of the study population was enriched by virtue of payor and insurance requirements,38 our study examined PAP adherence essentially among all-comers willing to engage with PAP treatment. Many of our patients have no or minimal out-of-pocket costs associated with their sleep studies, PAP equipment, and clinic visits, which greatly reduces financial barriers that would otherwise be encountered outside the VA healthcare system. Such a financially optimized system permits the examination of adherence above and beyond usual cost factors. Furthermore, we were able to adjust for patients’ socioeconomic status, which can mediate the outcomes seen in studies of minority health, by using a validated and comprehensive metric of neighborhood disadvantage.33 Additionally, it may be ideal to determine the relationship between black race and OSA treatment in veterans given that a greater proportion of individuals identify as black or African American in the veteran population compared to the general United States population (52% versus 32%).39
Our study has some limitations. Because our patients consisted predominantly of male veterans, our conclusions may not be generalizable to all individuals with OSA. Also, we did not examine PAP adherence over a longer time period. Multiple studies, however, have shown correlation between adherence from as early as 3–7 days with later adherence at 1, 3, and 6 months, and 1 year.23,40–42 Finally, although we identified an association between black race and PAP adherence, the retrospective study design did not allow us to determine the basis for the observed relationship. Studies have shown a higher prevalence of insomnia and short sleep duration among black adults,43,44 and it is unknown if these factors or other confounders explain the racial disparity that was seen. We also do not have data on important behavioral determinants of PAP use, such as self-efficacy, knowledge, and social support.
CONCLUSION
Race is a known contributor to health disparities. This study is the largest to date that examines the relationship between black race and PAP adherence in the treatment of obstructive sleep apnea, while adjusting for socioeconomic status and utilizing objective cloud-based adherence data. We have shown that black race is associated with reduced PAP adherence among veterans with OSA. The basis for this relationship needs to be explored in future studies so that steps can be taken to reduce racial health inequalities in the treatment of OSA.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Veterans Affairs Greater Los Angeles Healthcare, Los Angeles, CA. This material is based upon work supported by the ASPIRE (Academic Sleep Pulmonary Integrated Research/Clinical) Fellowship (N.H.). This material is based upon work supported by the National Institute on Aging of the National Institutes of Health under Award Number K23AG045937 and The Beeson Career Development in Aging Research Award Program (supported by NIA, AFAR, The John A. Hartford Foundation, and The Atlantic Philanthropies) (C.F). The authors report no conflicts of interest. The views expressed in this manuscript represent those of the authors and do not necessarily reflect those of the Department of Veterans Affairs or National Institutes of Health. Data from this study was previously published in abstract form in the abstract supplement issue of journal Sleep (Hsu et al. Association of black race with positive airway pressure therapy adherence among veterans with obstructive sleep apnea. Sleep. 2019;42(suppl_1):A210. https://doi.org/10.1093/sleep/zsz067.523; published by Oxford University Press on behalf of the Sleep Research Society) and presented at the annual meeting of the Associated Professional Sleep Societies (SLEEP) in June 2019, San Antonio, Texas.
SUPPLEMENTARY MATERIAL
ABBREVIATION
- ADI
Area deprivation index
REFERENCES
- 1.Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. 10.5664/jcsm.27497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–513. 10.1164/rccm.200909-1423OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. 10.1016/S0140-6736(08)61622-0 [DOI] [PubMed] [Google Scholar]
- 4.Garbarino S, Guglielmi O, Sanna A, Mancardi GL, Magnavita N. Risk of occupational accidents in workers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2016;39(6):1211–1218. 10.5665/sleep.5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–2176. 10.1001/jama.2012.3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez-García MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415. 10.1001/jama.2013.281250 [DOI] [PubMed] [Google Scholar]
- 7.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166(2):159–165. 10.1164/rccm.2105124 [DOI] [PubMed] [Google Scholar]
- 8.Venkatesh AK, Agha L, Abaluck J, Rothenberg C, Kabrhel C, Raja AS. Trends and variation in the utilization and diagnostic yield of chest imaging for medicare patients with suspected pulmonary embolism in the emergency department. AJR Am J Roentgenol. 2018;210(3):572–577. 10.2214/AJR.17.18586 [DOI] [PubMed] [Google Scholar]
- 9.Colvonen PJ, Masino T, Drummond SP, Myers US, Angkaw AC, Norman SB. Obstructive sleep apnea and posttraumatic stress disorder among OEF/OIF/OND veterans. J Clin Sleep Med. 2015;11(5):513–518. 10.5664/jcsm.4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Census Bureau. 2018 American Community Survey 5-Year Estimates. Accessed May 21, 2020. https://data.census.gov/cedsci/table?q=Populations%20and%20People&tid=ACSST5Y2017.S2101&t=Populations%20and%20People%3AVeterans&vintage=2018.
- 11.Schwab RJ, Badr SM, Epstein LJ, et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188(5):613–620. 10.1164/rccm.201307-1282ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indications and standards for use of nasal continuous positive airway pressure (CPAP) in sleep apnea syndromes. American Thoracic Society. Official statement adopted March 1944. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1738–1745 [DOI] [PubMed] [Google Scholar]
- 13.Rauscher H, Popp W, Wanke T, Zwick H. Acceptance of CPAP therapy for sleep apnea. Chest. 1991;100(4):1019–1023. 10.1378/chest.100.4.1019 [DOI] [PubMed] [Google Scholar]
- 14.Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365–369. 10.1155/2008/534372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldhorn RE, Herrick TW, Nguyen MC, O’Donnell AE, Sodero J, Potolicchio SJ. Long-term compliance with nasal continuous positive airway pressure therapy of obstructive sleep apnea. Chest. 1990;97(1):33–38. 10.1378/chest.97.1.33 [DOI] [PubMed] [Google Scholar]
- 16.Billings ME, Auckley D, Benca R, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34(12):1653–1658. 10.5665/sleep.1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagnadoux F, Le Vaillant M, Goupil F, et al. Influence of marital status and employment status on long-term adherence with continuous positive airway pressure in sleep apnea patients. PLoS One. 2011;6(8):e22503. 10.1371/journal.pone.0022503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joo MJ, Herdegen JJ. Sleep apnea in an urban public hospital: assessment of severity and treatment adherence. J Clin Sleep Med. 2007;3(3):285–288. 10.5664/jcsm.26800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platt AB, Field SH, Asch DA, et al. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32(6):799–806. 10.1093/sleep/32.6.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. 10.1016/j.smrv.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valentin A, Subramanian S, Quan SF, Berry RB, Parthasarathy S. Air leak is associated with poor adherence to autoPAP therapy. Sleep. 2011;34(6):801–806. 10.5665/SLEEP.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419–426. 10.1111/j.1365-2869.2011.00969.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budhiraja RPS, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–324. [PubMed] [Google Scholar]
- 24.Guralnick AS, Pant M, Minhaj M, Sweitzer BJ, Mokhlesi B. CPAP adherence in patients with newly diagnosed obstructive sleep apnea prior to elective surgery. J Clin Sleep Med. 2012;8(5):501–506. 10.5664/jcsm.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Means MK, Ulmer CS, Edinger JD. Ethnic differences in continuous positive airway pressure (CPAP) adherence in veterans with and without psychiatric disorders. Behav Sleep Med. 2010;8(4):260–273. 10.1080/15402002.2010.509255 [DOI] [PubMed] [Google Scholar]
- 26.Scharf SM, Seiden L, DeMore J, Carter-Pokras O. Racial differences in clinical presentation of patients with sleep-disordered breathing. Sleep Breath. 2004;8(4):173–183. 10.1055/s-2004-860894 [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, Chakraborty A, Chowdhury A, Mukhtar U, Willes L, Quan SF. Adherence to positive airway pressure therapy in hospitalized patients with decompensated heart failure and sleep-disordered breathing. J Clin Sleep Med. 2016;12(12):1615–1621. 10.5664/jcsm.6344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace DM, Shafazand S, Aloia MS, Wohlgemuth WK. The association of age, insomnia, and self-efficacy with continuous positive airway pressure adherence in black, white, and Hispanic U.S. Veterans. J Clin Sleep Med. 2013;9(9):885–895. 10.5664/jcsm.2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace DM, Wohlgemuth WK. Does race-ethnicity moderate the relationship between CPAP adherence and functional outcomes of sleep in US veterans with obstructive sleep apnea syndrome?. J Clin Sleep Med. 2014;10(10):1083–1091. 10.5664/jcsm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96(12):2113–2121. 10.2105/AJPH.2005.077628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99(9):1013–1023 [PMC free article] [PubMed] [Google Scholar]
- 32.Darin-Mattsson A, Fors S, Kareholt I. Different indicators of socioeconomic status and their relative importance as determinants of health in old age. Int J Equity Health. 2017;16(1):173. 10.1186/s12939-017-0670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kind AJH, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization. Ann Intern Med. 2014;161(11):765. https://doi.org/10.7326/M13-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93(7):1137–1143. 10.2105/AJPH.93.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durfey SNM, Kind AJH, Buckingham WR, DuGoff EH, Trivedi AN. Neighborhood disadvantage and chronic disease management. Health Serv Res. 2018;54(Suppl 1):206–216. 10.1111/1475-6773.13092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu J, Kind AJH, Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual. 2018;33(5):493–501. 10.1177/1062860617753063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz SW, Sebastiao Y, Rosas J, Iannacone MR, Foulis PR, Anderson WM. Racial disparity in adherence to positive airway pressure among US veterans. Sleep Breath. 2016;20(3):947–955. 10.1007/s11325-016-1316-1 [DOI] [PubMed] [Google Scholar]
- 38.Cistulli PA, Armitstead J, Pepin JL, et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. 2019;59114–116. 10.1016/j.sleep.2019.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Office of Data Governance and Analytics. Minority Veterans Report: military service history and VA benefit utilization statistics. March 2017. Department of Veterans Affairs, Washington, DC. Accessed April 11, 2020. https://www.va.gov/vetdata/docs/SpecialReports/Minority_Veterans_Report.pdf.
- 40.Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5(3):229–240. 10.1080/15402000701264005 [DOI] [PubMed] [Google Scholar]
- 41.Colvin LJ, Dace GA, Colvin RM, Ojile J, Collop N. Commercial motor vehicle driver positive airway pressure therapy adherence in a sleep center. J Clin Sleep Med. 2016;12(4):477–485. 10.5664/jcsm.5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. 10.1093/sleep/20.4.278 [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. 10.1093/sleep/30.9.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.