Scheme 3. Benzylic C–H Cross-Coupling to C–O, C–N, and C–C Bonds via a Benzyl Fluoride.
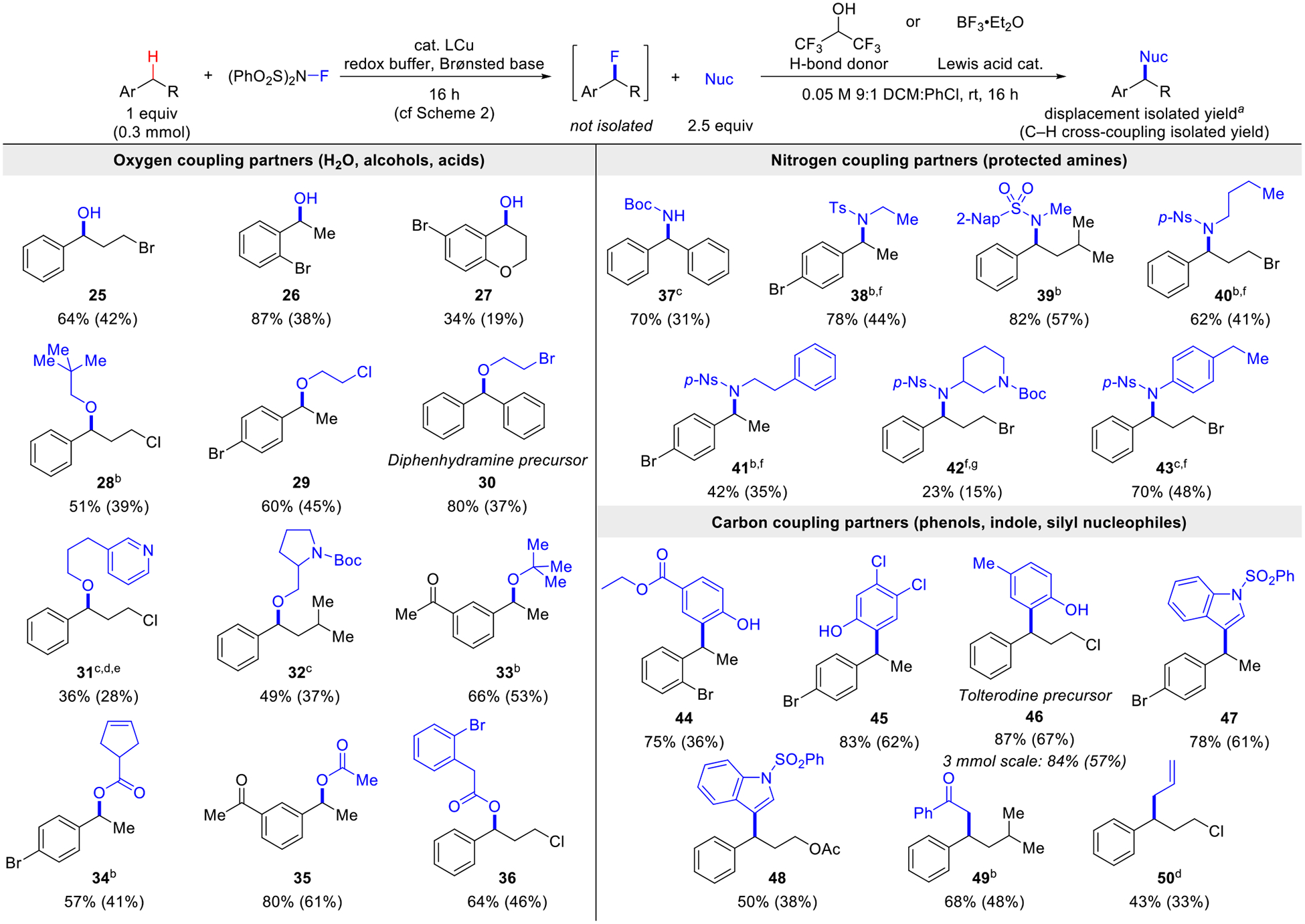
aReaction uses 10 equiv HFIP as a H-bond donor. Isolated yields calculated with respect to the 1H NMR yield of the benzyl fluoride (or the C–H substrate, in parentheses). b10 mol% BF3•Et2O used instead of HFIP. c50 mol% BF3•Et2O used instead of HFIP dBoth HFIP and BF3•Et2O used. e2.5 equiv MsOH added to the nucleophile. fUsed dichloromethane as the fluorination reaction solvent. g1.5 equiv BF3•Et2O used instead of HFIP. Isolated as the amine.
