Fig. 2.
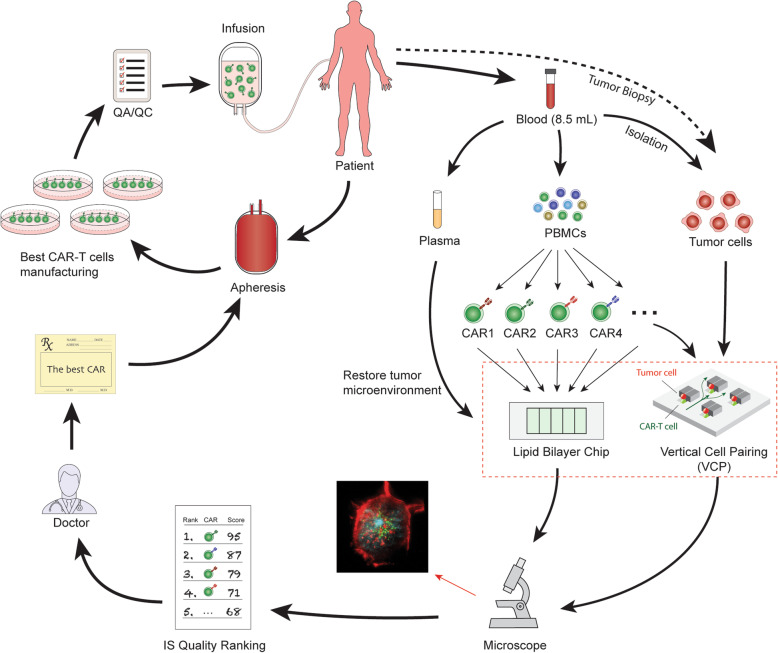
Select the best CAR from different company products for a particular patient. Blood is collected by a lab professional into a 10 ml tube with anticoagulant as a regular specimen collection procedure in any certified blood testing laboratory. The tube can be sent directly to the synapse testing lab without transferring to a secondary tube. PBMCs, plasma, and tumor cells can be enriched. Plasma can be used later for mimicking tumor microenvironment by adding the patient’s plasma to the imaging system on both the lipid bilayer system and VCP system. Meanwhile, the enriched PBMCs are placed in culture and expanded. The viral vectors containing CAR1, CAR2, CAR3, CAR4, etc. are added to generate different versions of CAR products. These different CAR products are subjected to IS quality testing. Both the lipid bilayer chip and VCP device can be used to evaluate IS quality. IS quality ranking reports can be presented to physicians who can use the data to prescribe the best CAR product for a particular patient. A manufacturing company then generates this prescribed CAR with proper quality control release testing and quality assurance review. The final product is cryopreserved and delivered to distant infusion sites, where the CAR T medicine is infused