Figure 1. Fundamental insights into Cu/NFSI-catalyzed fluorination reactions.
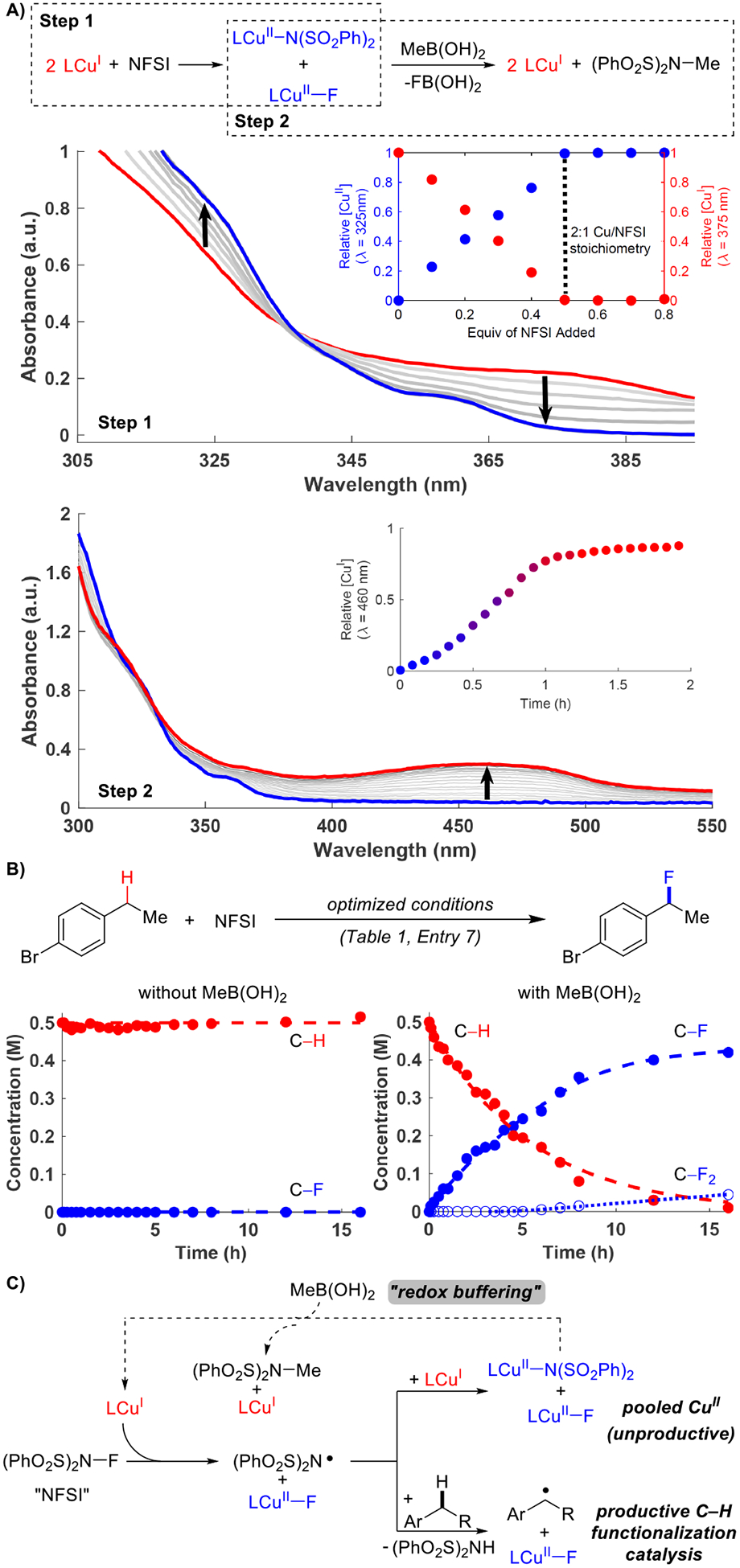
A) Spectroscopic analysis of stoichiometric oxidation of CuI by NFSI and reduction of CuII by MeB(OH)2. B) Reaction time course data demonstrating the effect of MeB(OH)2 redox buffering in the catalytic fluorination reaction. C) Mechanism depicting the redox buffering role of MeB(OH)2 in benzylic fluorination. Conditions: A) Step 1 – 0.33 mM BPhenCuIOAc + 0.1 equiv NFSI (×8) in PhCl. Step 2 – [CuII] (from 0.33 mM BPhenCuI(OAc) + 0.5 equiv NFSI) + 5 equiv MeB(OH)2, 15 equiv Li2CO3 in PhCl. B) See Table 1, entry 7.
